Q & A

| Q & A |
 |
|
|
September 2012 Editor:
A. The mean corpuscular hemoglobin (MCH) still has a place in clinical diagnosis. MCH is derived from the ratio of total hemoglobin to the erythrocyte count. With the hemoglobin expressed as g/L and the erythrocyte count expressed as × 1012/L, the results are presented in picograms (that is, 10-12 g). Therefore, MCH provides an absolute gravimetric measurement of the hemoglobin in the average erythrocyte. 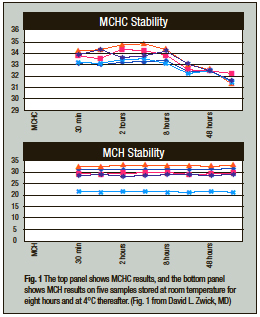
MCHC, however, provides the ratio of the hemoglobin to the volume of the average cell. MCHC is useful in many conditions, such as normochromic normocytic anemias, where the amount of hemoglobin produced parallels changes in the MCV. However, when evaluating conditions with a decreased erythrocyte volume, such as thalassemia or iron deficiency, or increased absolute erythrocyte hemoglobin such as hemochromatosis, changes in erythrocyte volumes create problems in interpretation if the MCV or MCHC are used. For example, as erythrocytes age, the MCV will increase and consequently (as shown by the data from David L. Zwick, MD, Fig. 1) the MCHC will decrease, yet the absolute amount of MCH does not change. Such constancy of the MCH is why decreased MCH is used as a tool for evaluating possible thalassemia, iron deficiency, or hemochromatosis.1-3 References 1. Ryan K, Bain BJ, Worthington D, et al. Significant haemoglobinopathies: guidelines for screening and diagnosis. Brit J Haematol. 2010;149:35–49. 2. Pranpanus S, Sirichotiyakul S, Srisupundit K, et al. Sensitivity and specificity of mean corpuscular hemoglobin (MCH): for screening alpha-thalassemia-1 trait and beta-thalassemia trait. J Med Assoc Thai. 2009;92:739–743. 3. Barton JC, Bertoli LF, Rothenberg BE. Screening for hemochromatosis in routine medical care: an evaluation of mean corpuscular volume and mean corpuscular hemoglobin. Genet Test. 2000;4:103–110. David F. Keren, MD, Professor of Pathology
A. Point-of-care PT/INR testing is FDA approved for warfarin monitoring. Once the patient’s dose requirements and anticoagulation effects have been stabilized for one to two weeks using a central laboratory, testing can be moved to a POC testing device. In this setting, the testing is CLIA waived. POC PT/INR devices have not been approved for coagulation screening, testing for DIC, or for use in the trauma/emergency department setting. Central laboratory testing with a more expanded coagulation panel, to include at least PT, PTT, FSP, D-dimer, CBC, and anti-thrombin testing, is indicated in these situations. POC PT/INR testing is limited to the stabilized patient for warfarin monitoring. Use of POC PT/INR devices for other than warfarin monitoring requires proof of medical necessity and a separate validation study, and it would be considered a high-complexity test. Off-label device use could potentially lead to patient mismanagement and unexpected consequences. Bibliography 1. Centers for Disease Control and Prevention. Clinical Laboratory Improvement Amendments, Subpart A, Section 493.5 and 493.15. wwwn.cdc.gov/clia/regs/subpart_a.aspx#493.5. Accessed Feb. 22, 2012. 2. Centers for Disease Control and Prevention. Good Laboratory Practices for Waived Testing Sites. www.cdc.gov/mmwr/preview/mmwrhtml/rr5413a1.htm. Accessed Feb. 22, 2012. 3. Centers for Disease Control and Prevention. Waived Tests. wwwn.cdc.gov/dls/waivedtests/. Accessed Feb. 22, 2012. 4. College of American Pathologists. Point of Care Testing Toolkit. Pathologist’s Regulatory Role in Addition to CLIA. www.cap.org, CAP Committees and Leadership>Point of Care Testing Topic Center>Point of Care Testing Toolkit. Accessed Feb. 22, 2012. 5. U.S. Food and Drug Administration. Follow Good Laboratory Practices with Waived Laboratory Tests. www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ Deborah A. Perry, MD
A. The AMR (analytic measurement range) is defined as the “… range of analyte values that a method can directly measure on the specimen without any dilution, concentration, or other pretreatment not part of the usual assay process.”1 For FDA-approved tests, the vendor will provide the AMR for each test as part of the test procedure. It is not unusual for a test result to fall outside of the AMR and for laboratories to report such a result exceeding the AMR as < or > AMR. There is no recommended maximum dilution for any analyte. Each laboratory should determine on an analyte-by-analyte basis what, if any, level of maximum dilution should be applied. In general, specimen results that fall outside the AMR may be unreliable because the manufacturer has not validated the assay for results that exceed the AMR. The CAP checklist requirement (CHM.13720)1 says that if a dilution/concentration protocol is used, then it should be defined on an analyte-specific basis in the procedure manual with medical director approval. At our institutions, values outside the AMR are reported simply as > or <, which is sufficient to establish their clinical significance. For a limited number of analytes, we do have dilution protocols should the clinician specifically request one. References 1. College of American Pathologists Commission on Laboratory Accreditation. Chemistry and Toxicology Checklist. July 31, 2012 edition. Northfield, Ill.: College of American Pathologists. 2012. David N. Alter, MD Dr. Kiechle is medical director of clini-cal pathology, Memorial Healthcare, Hollywood, Fla. |