July 1999
Coagulation Case Study
Robert B. Fairweather, MD, PhD
Third in a periodic series of articles, written by members of the CAP Coagulation Resource Committee, focusing on laboratory evaluation of coagulation disorders.
Clinical history. An 80-year-old man with a medical history significant for atherosclerotic coronary vascular disease, mild hypertension, and mild, chronic renal failure was admitted to the hospital for elective total hip replacement. The patient was given a low-molecular-weight heparin-30 mg by subcutaneous injection-peri-operatively twice a day for prophylaxis against deep venous thrombosis.
On hospital day four, the patient developed pneumonia and was treated with appropriate antibiotics. He subsequently developed progressive respiratory failure and was transferred to an intensive care unit, where he underwent intubation on day 15. The patient continued to receive prophylactic LMWH, which was increased on day 23 to a treatment dose of 60 mg (approximately 1 mg/kg) by subcutaneous injection twice a day for suspected deep vein thrombosis.
On day 28, the patient abruptly developed an extensive hematoma of the entire left upper extremity, oozing blood from vascular access lines, and his hemoglobin decreased by 2.5 g/dL during a 24-hour period. Results of screening coagulation tests ordered by the attending physician at that time are presented in Table 1.

Laboratory test-based algorithm. The differential diagnosis of causes of acute onset of bleeding with prolonged prothrombin time, activated partial thromboplastin time, and thrombin time are presented in Fig. 1. Based on the differential diagnosis and clinical history, the laboratory director requested or reviewed additional laboratory tests. The results are presented in Table 2.
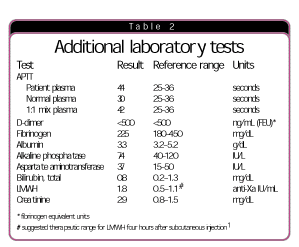
The 1:1 APTT mixing study does not correct, suggesting inhibition of the test (antibody or medication) rather than factor deficiency, as would be seen in a consumptive process. The negative D-dimer and normal fibrinogen may rule out disseminated intravascular coagulation and hypofibrinogenemia. Liver disease, including associated dysfibrinogenemia, is unlikely based on a negative clinical history and normal liver function tests. The patient had not been exposed to topical bovine thrombin during any surgery. Furthermore, repeating the thrombin time using human thrombin provided the same result as using bovine thrombin. Factor VIII inhibitor might need to be considered. In the usual case of specific inhibitor to factor VIII, only the APTT will be prolonged. However, in hospitalized patients, other conditions may complicate the picture. Vitamin K deficiency in patients who have been hospitalized for lengthy periods may cause hemorrhage or complicate other bleeding disorders, particularly if the patients depend on parenteral nutrition. However, vitamin K deficiency alone would not account for a prolonged thrombin time. The approach to vitamin K deficiency was addressed in a previous coagulation case study. (See “Identifying vitamin K deficiency as the culprit in coagulation abnormality,” CAP TODAY, January 1999, page 38.) Each of the other disorders listed in Fig. 1 will be addressed in detail in future installments.

Laboratory professionals identified the supratherapeutic LMWH level as the cause of the patient’s bleeding and mildly prolonged coagulation tests. The patient’s physician discontinued LMWH, and the plasma level decreased. Based on sequential assays in this patient, which followed a first order exponential decay (Table 3), the elimination half-life appeared to be prolonged to approximately 25 hours. The bleeding stopped without further complication, and the patient was discharged to an extended care facility on day 49.

Discussion. Low-molecular- weight heparins are prepared from unfractionated heparin by chemical or enzymatic depolymerization. Several LMWH preparations have been approved by the Food and Drug Administration for deep vein thrombosis prophylaxis during orthopedic and abdominal surgery, and one recently has been approved for use in the treatment of deep vein thrombosis. Low-molecular-weight heparin offers several advantages over unfractionated heparin.1 Unlike unfractionated heparin, LMWHs have excellent bioavailability as a result of reduced nonspecific binding to plasma proteins and endothelium. Pharmacokinetics are more uniform, leading to a predictable response in most patients without the need for laboratory monitoring in most situations. LMWH is cleared primarily by renal excretion; with normal renal function, the elimination half-life is longer than that of unfractionated heparin and varies from two to six hours, depending on the route of administration. This allows one to achieve adequate plasma levels by subcutaneous administration once or twice daily. The incidence of heparin-induced thrombocytopenia also appears to be lower with LMWH.
LMWH, in conjunction with antithrombin, acts by inhibiting factor Xa. However, it does not significantly prolong most screening coagulation tests. This is the result of a lower inhibitory activity against thrombin (IIa) compared with unfractionated heparin. The greater sensitivity of the APTT to unfractionated heparin may be due to inhibition of the feedback activation of factors V and VIII by thrombin. Direct inhibition of thrombin by unfractionated heparin dramatically prolongs the thrombin time. Furthermore, most thromboplastin reagents contain heparin-neutralizing agents, which accounts for the lack of sensitivity of the prothrombin time to heparin or LMWH. Most LMWHs have an anti-Xa:anti-IIa ratio between 2 and 4, compared with approximately 1 for unfractionated heparin.
Plasma concentration of LMWH is most easily measured by its ability to inhibit exogenous factor Xa in an anti-Xa activity assay. Several commercial formulations are available, and most employ a chromogenic method. It is recommended that the LMWH preparation used for assay calibration be an international standard or a LMWH linked to that standard.1 If administered subcutaneously, peak plasma concentrations occur three to five hours after injection. One generally is advised to collect a plasma sample four hours after injection. The therapeutic range for LMWH has not been rigorously defined. For treating venous thromboembolism, with twice daily dosing, an acceptable range for a sample collected four hours after subcutaneous injection (peak level) is 0.5 to 1.1 anti-Xa U/mL.1
Although laboratory monitoring of LMWH generally is not required, it may be indicated for some patients to prevent excessive or insufficient anticoagulation. Fig. 2 presents an approach to evaluating a patient who is to be treated with LMWH. Infants and small children may require a larger dose of the drug than adults. Studies have not yet confirmed that unit dosing is safe and effective in children, so monitoring is recommended to ensure adequate therapy. Patients who are obese or have low body weight may require intermittent monitoring because of possible differences in their pharmacokinetics compared with patients closer to ideal body weight. Because the kidney primarily clears the drug, patients with renal insufficiency may benefit from periodic monitoring. The elimination half-life may increase several-fold, as was seen in this case. Monitoring may be useful in outpatients on long-term therapy for conditions such as malignancy (Trousseau’s syndrome) and thrombosis refractory to oral anticoagulants (as in myeloproliferative disorders and antiphospholipid antibody syndrome), as well as in individuals who cannot take oral anticoagulants, such as pregnant patients or those who have allergic reactions to the drug.

One can manage patients with bleeding complications from LMWH therapy by withholding the drug or transfusing red blood cells and volume expanders-however, fresh frozen plasma will not be useful because of the inhibitory activity of the drug. Physicians should consider treating with protamine sulfate to neutralize the heparin if bleeding is excessive or blood pressure falls.
LMWH can cause heparin-induced thrombocytopenia, although this is much less common than with unfractionated heparin. A platelet count prior to treatment will be useful when evaluating the patient who has complications and is found to be thrombocytopenic. It may not be necessary to monitor the platelet count routinely in most patients.
This case illustrates several problems related to managing LMWH in the “atypical” patient and emphasizes the following points:
Reference
1. Laposata M, Green D, Van Cott EM, et al. College of American Pathologists Conference XXXI on laboratory monitoring of anticoagulant therapy. The clinical use and laboratory monitoring of low-molecular-weight heparin, danaparoid, hirudin, and related compounds, and argatroban. Arch Pathol Lab Med. 1998;122:799-807.
Dr. Fairweather is associate professor and chief of clinical pathology section, Dartmouth-Hitchcock Medical Center, Lebanon, NH. He is a member of the CAP Coagulation Resource Committee.