September 1999
Coagulation Case Study
John D. Olson, MD, PhD
This is the fourth in a periodic series of articles written by members of the CAP Coagulation Resource Committee and focusing on laboratory evaluation of coagulation disorders
Clinical history. A 42-year-old woman who was the victim of a motor vehicle accident presented to a hospital emergency room. She was conscious at the time of presentation. Following an immediate imaging evaluation, she was taken to the operating room for open reduction of multiple fractures of the left hip and femur. She received four units of packed red blood cells intraoperatively.
During the postoperative period, after her admission to the surgical intensive care unit, the patient’s vital signs were stable and laboratory test results were obtain-ed (Table 1). The laboratory findings led to the evaluation of the patient's prolonged activated partial thromboplastin time.
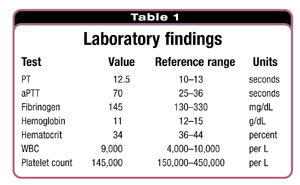
At the beginning of the algorithm for determining the etiology of an abnormal aPTT at the hospital, the thrombin clotting time was longer than 150 seconds. This raised the possibility that the specimen was contaminated by heparin. Consultation with the nursing staff in the intensive care unit confirmed that the specimen had been drawn from an arterial line that was"kept open" with heparin. Repeat coagulation studies performed on a specimen collected from a peripheral vein revealed an aPTT within the reference range.
This brief clinical history provides an introduction to a laboratory evaluation of a prolonged aPTT. The aforementioned clinical situation is relatively common. Despite efforts to flush heparin from central venous lines, heparin contamination of coagulation specimens drawn from such lines continues to be a problem.
Since it was first described in 1953, the partial thromboplastin time has been a valuable tool for monitoring heparin therapy and evaluating patients who have a suspected bleeding disorder. Methods for automating the reading of the end point for the prothrombin time and aPTT were developed in the 1960s and early 1970s. At the same time, manufacturers began to provide reagents for performing the tests. In the 1970s, the PTT method changed. Kaolin, celite, micronized silica, or elagic acid were added to the test to activate contact factors and change the PTT to aPTT. This is the method that prevails today. The responsiveness of the reagents used for the aPTT continue to vary widely in all clinical uses.
Before addressing clinical etiologies of a prolonged aPTT, it is important to note that several preanalytical (spurious) factors can cause an abnormal time. Among them are an unusually high or low hematocrit causing an alteration in the concentration of citrate, con-tam-ination of the specimen with EDTA, and formation of clots in the specimen. One must be able to exclude these and other spurious causes when evaluating any coagulation test.
Prolongation of the aPPT typically can be attributed to five common causes (Table 2). Each of the different etiologic categories for a prolonged aPTT requires a specific action on the part of the clinician who responds to the abnormal test result. The following are of clinical significance:

1. Medication –aPTT is the test most often used for monitoring therapy with unfractionated heparin, which can contaminate specimens for coagulation testing that are drawn inappropriately from arterial or central venous lines. When used to monitor heparin therapy, the aPTT can be of value in terms of adjusting dosage. Contamination of a specimen by heparin can lead to unnecessary and costly additional laboratory work and, if not detected promptly, to inappropriate testing. Furthermore, it can obfuscate the interpretation of those tests.
Although heparin is the medication most likely to prolong the aPTT, new medications, such as hirudin analogues and argatroban, are beginning to be used in clinical situations and can inhibit thrombin and produce a prolonged aPTT. Other less frequent causes of such prolongation include the presence of hydroxy-ethyl starch, hematin, and Suramin. In addition, Taularidine, an additive in some intravenous medications, can lengthen the aPTT.
It is important to exclude early a medication etiology for a prolonged aPTT. This may require using the thrombin clotting time or adding heparinase to the specimen to interpret the result correctly.
2. Coagulation factor deficiencies associated with significant hemorrhage–Inherited deficiencies of not only the most commonly deficient coagulation factors–factors VIII, IX, and XI–but also other coagulation factors can significantly prolong the aPTT. In addition, acquired deficiencies of individual or multiple coagulation factors, as might be seen in patients with liver disease or consumption coagulopathy, can prolong an aPTT. The latter are frequently accompanied by a prolonged PT. When coagulation factors are severely deficient, a clinician often must replace those deficient factors or, in the case of acquired deficiencies, treat the underlying etiology of the factor deficiency.
3. Coagulation factor deficiencies of little or no clinical significance–Most medical students are taught about the intrinsic and extrinsic activation of the common pathway of coagulation. This approach to thinking about the mechanism of thrombin generation has emanated from our knowledge about the activation processes that occur in vitro.
Activation of coagulation in vivo proceeds differently and involves considerable cross activation between the intrinsic and extrinsic systems. Because of this difference between the in vivo and in vitro activation processes, patients may have significant deficiencies of coagulation factors involved in contact activation (factor XII, prekallikrein, and high-molecular-weight kininogen) that lead to a markedly prolonged aPTT that has no associated bleeding diathesis. Such deficiencies generally are of no consequence.
There have been infrequent reports of patients with severe deficiencies of contact factors who have hypercoagulability. This is presumed to be the consequence of these contact factors activating fibrinolysis, which, when reduced, can lead to hypercoagulability. In general, a prolonged aPTT caused by deficiency of factor XII, prekallikrein, or high-molecular-weight kininogen is a nuisance and of no clinical consequence.
4. Nonspecific (lupus-type) anticoagulant (LA)–A previous coagulation case study detailed the evaluation of antiphospholipid antibodies and LAs. (See"Diagnosing antiphospholipid antibody syndrome," CAP TODAY, March 1999, page 84.) Lupus anticoagulants can significantly prolong the aPTT that usually fails to correct in a mixing study. Demonstrating that this anticoagulant activity is dependent on phospholipid confirms the diagnosis of an LA. The patient who has an LA should be evaluated for a hypercoagulable state and a propensity for thrombosis. If an LA is found in a patient with thrombosis, it may be necessary to prolong, and possibly increase, the intensity of anticoagulation therapy.
5. Specific coagulation factor inhibitor–Approximately 15 percent of patients who have severe factor VIII or severe factor IX deficiency develop alloantibodies that recognize the deficient factor when it is transfused. Furthermore, patients may spontaneously develop autoantibodies that recognize and rapidly clear their own coagulation factors. The vast majority of these specific autoantibodies that lead to profound hemorrhage are directed at the factor VIII molecule. It is critical that clinicians be able to recognize such an inhibitor because the hemorrhage may be life-threatening and it may be extremely difficult to manage the patient.
Laboratory test-based algorithm. It is important to evaluate a new reagent or an instrument used to perform the aPTT in order to determine the effectiveness of the method in identifying the aforementioned etiologies. The validation of a new aPTT method will require: determining its sensitivity to heparin and, possibly, other drugs, and its sensitivity to a factor deficiency, particularly factors VIII and IX; determining how the aPTT reagent performs in evaluating the mixing study in factor-deficient plasmas and plasmas containing anticoagulant; and determining the sensitivity of the reagent to an LA.
An algorithm for evaluating a prolonged aPTT appears in Fig. 1. It is not the only clinically acceptable algorithm, but it is one approach that may be helpful in sorting the five potential clinical outcomes. The algorithm is described briefly below.

In most cases, laboratory professionals will know the value of the fibrinogen by the time they evaluate a prolonged aPTT. If this value is not known, we find it useful, in our laboratory, to determine that a fibrinogen concentration is sufficient to support the test. (It is frustrating to fully evaluate a prolonged aPTT only to find that the patient suffers from hypofibrinogenemia.) If the fibrinogen concentration is sufficient, it is useful to conduct an evaluation to exclude heparin or other medications.
Our laboratory has found that the use of a sensitive thrombin time (control of 18 to 20 seconds) can be helpful. With most methods, if there is sufficient heparin in the specimen to prolong the aPTT beyond the reference range by only one second, the thrombin time will be longer than 150 seconds. Therefore, the finding of a measurable thrombin time excludes heparin as the etiology of a significantly prolonged aPTT. A mixing study is useful if the thrombin time is measurable.
A recent CAP proficiency testing questionnaire queried a large number of laboratories about method and reagent selections for PT and aPTT mixing studies. The results of that query demonstrated wide variability in approaches to performing the mixing study. A manuscript that summarizes the findings is being prepared. It will provide recommendations for performing the mixing study. A few points are worth addressing here:
The approach to the mixing study described herein involves mixing an equal volume of the patient's plasma and the control plasma and immediately performing the aPTT. If the resulting aPTT fails to correct to the reference range, an inhibitor likely is present and evaluation of the type (cause) of the inhibitor is indicated. If the value corrects into the reference range, one must perform an incubated mix to determine if a time-dependent inhibitor is present.
Performing an incubated mix (Fig. 2) involves mixing an equal volume of the patient's plasma and the pooled normal plasma and incubating the mixed specimen (tube A). This becomes the test specimen. To form the control specimen, the patient specimen (tube B) and the pooled normal plasma (tube C) are incubated for the same time (one to two hours; 37 º C). After incubation, equal parts of tubes B and C are mixed (tube D). At the end of the incubation, a laboratory professional measures the aPTT of the mixed specimens (tube A, the test, and tube D, the control). If tubes A and D produce the same aPTTs, an interpretation of a corrected mixing study is made and an evaluation for factor deficiency would be indicated.
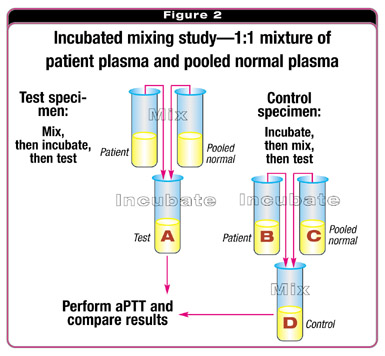
The patient's clinical history will determine whether one initially would evaluate contact activation factors or procoagulant factors. If the test specimen is prolonged when compared with the control specimen, one can interpret that the mix failed to correct and an inhibitor is present. The next step is to determine whether the inhibitor is phospholipid-dependent. A variety of tests are available to determine phospholipid dependence of inhibition, and more than one of these should be available for the evaluation because none are sufficiently sensitive or specific to the presence of an LA. If phospholipid dependence is determined, and the patient's clinical history is consistent with this determination, the diagnosis is an LA. If phospholipid dependence is not found, the patient should be evaluated for the presence of a specific coagulation inhibitor.
Conclusion. Although simplified, this algorithm offers an approach to evaluating a prolonged aPTT. As one performs the evaluation, it is important to consider the following:
Mixing studies are as much an art form as a science and must be interpreted by laboratory professionals who have intimate knowledge of the test performance in their laboratories.
Dr. Olson is professor and director of Clinical Laboratories, Department of Pathology, University of Texas Health Science Center, San Antonio. He is chair of the CAP Coagulation Resource Committee.