March 2024—Like identifying the shift in battle that leads to victory, or the battle that wins the war—let alone declaring a war’s ultimate victor—it’s hard to gauge the whens, ifs, and hows that mark progress in medicine. For those who are deeply rooted in bringing advances to testing in urothelial cancers, current research is flourishing and flummoxing. In early and late stage, both for bladder and upper tract disease, recently approved therapies are leading to better outcomes for patients. More immunotherapies and antibody-drug conjugates are on their way, and with them come new options for testing. But as with any cancer, researchers follow numerous promising paths, knowing that some will dead-end and others will succeed primarily (albeit usefully) in raising more questions. Nevertheless, they continue to rally the work forward, with multiple breaches, and Agincourt, ever in sight. For experts such as David McConkey, PhD, progress will best be measured by how regularly precision makes its way into the clinical setting.
Read More »ARTICLES
From training to first jobs, can the transition be made easier?
March 2024—Pathology trainees and training programs vary, as do first jobs, but the first year in pathology practice is generally said to be a tough one, largely because of the transition to fully independent case sign-out.
Read More »In diabetes patients, biomarker use for early-stage HF
March 2024—For patients with type 2 diabetes, the cardiac biomarkers are a better predictor of early-stage heart failure than conventional risk prediction scores. “We need to use biomarkers,” says Petr Jarolim, MD, PhD.
Read More »Survey probes staff shortage in genomics labs
March 2024—From a technologist workforce perspective, clinical genomics laboratories are in trouble. “It’s truly a crisis,” said Marco Leung, PhD, clinical director of the Steve and Cindy Rasmussen Institute for Genomic Medicine at Nationwide Children’s Hospital in Columbus, Ohio.
Read More »AP and CP reporting, from interfaces to IT wishes
March 2024—Anatomic and clinical pathology reporting—what’s working, what’s missing. Three pathologists (all board certified in informatics) and representatives of three information system companies met online Dec. 19 with CAP TODAY publisher Bob McGonnagle to talk about reporting needs and what’s optimal. The first half of their discussion was published in the February issue, with CAP TODAY’s guide to anatomic pathology computer systems. The second half begins here.
Read More »What’s going on? Interpreting urine toxicology cases
March 2024—For urine toxicology screening, immunoassays are automated and rapid but have variable sensitivity and specificity and results are considered presumptive. Mass spectrometry, used for confirmation, has superior sensitivity and specificity but is labor-intensive and slow and requires significant expertise.
Read More »AMP case report: Acute myeloid leukemia with hyperdiploidy
March 2024—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. This month's report comes from Aga Khan University in Karachi, Pakistan. Case. An 87-year-old male with a clinical history of hypertension and sick sinus syndrome presented with a one-month history of fever, generalized weakness, and weight loss. There was no lymphadenopathy or hepatosplenomegaly on physical examination. Bone marrow examination was performed to evaluate for cytopenias.
Read More »In urinalysis, compromises, collections, and rules
March 2024—Reflex criteria, middleware, bladder cancer screening, point of care, controls, and collections came up in CAP TODAY’s Jan. 16 roundtable on urinalysis. Six people weighed in, with CAP TODAY publisher Bob McGonnagle leading. Their take on where things stand and where they can be better follows. CAP TODAY’s guide to urinalysis instrumentation begins here. Tim Skelton, in last year’s ...
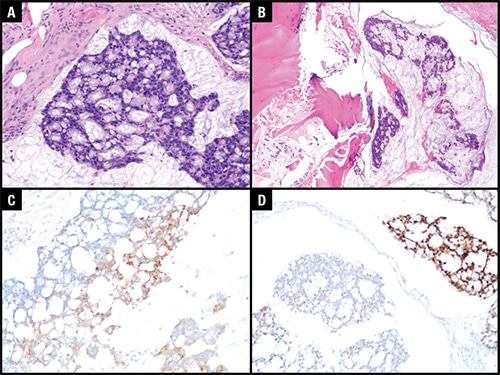
Read More »Doing more, doing better in bladder cancer
February 2024—From her vantage at the University of Texas MD Anderson Cancer Center, Donna Hansel, MD, PhD, has a clear view of cancer’s latest frontiers. Progress and breakthroughs are the norm. But even she sounds impressed when she surveys the changes in her specialty, urothelial cancer. “We are now thinking what we never before thought was possible: We are thinking about cures and lifelong remission from disease,” says Dr. Hansel, division head and professor of pathology and laboratory medicine. It’s been a long time coming, says Dr. Hansel, who is also the Dr. Eva Lotzova and Peter Lotz memorial research chair. The disease historically has been caught in a sort of prepositional triangle—underfunded, overlooked, and underdiagnosed—with serious consequences. For years, she says, “We thought bladder cancer had only one treatment”—BCG, or Bacillus Calmette-Guérin, therapy. Because the field lacked a large volume of research to propel better diagnostics and treatments, “people died of this disease because it progressed.”
Read More »As AI use expands, ethics at the leading edge
February 2024—Artificial intelligence is sizzling, so much so that New Yorker magazine, evoking the dazzling and the potentially devouring nature of AI technology, tagged 2023 as “The Year A.I. Ate the Internet.”
Read More »Biomarker tests with discrepant results—why the differences?
February 2024—When multimodality testing reveals discordant biomarker results, which method is correct? Annette S. Kim, MD, PhD, and JinJuan Yao, MD, PhD, in a CAP23 session last fall used their cases to share strategies for resolving discrepancies—or, in some cases, what look like discrepancies.
Read More »With pipeline for pathologists, others lacking, eyes on AI
February 2024—Artificial intelligence and Medicare Advantage contracts were at the center of the Jan. 2 Compass Group virtual roundtable led by CAP TODAY publisher Bob McGonnagle. “If you want to get into the AI world, there are many lanes you can swim in,” said Michael Feldman, MD, PhD, of Indiana University School of Medicine.
Read More »How we can help pathologists in Ethiopia
February 2024—Ethiopia’s health care system is at a crossroads as it grapples with the dual burden of infectious diseases and a surge in noncommunicable ailments.
Read More »Group’s pathology aides fill gaps, lighten the workload
February 2024—Efficiency can be hard to measure. But Kenneth Batts, MD, of Hospital Pathology Associates in Minneapolis, which contracts with Allina Health to provide anatomic pathology coverage, is sure that a pathology aide program the group started long ago makes its pathologists far more efficient.
Read More »The race to keep pace with drug use changes
February 2024—Xylazine prevalence, lab-developed testing, and new technology are converging at Yale New Haven Health in a way that gives rise to questions, worry, and new hope for faster drug testing.
Read More »AP and CP reporting—the needs, the caveats
February 2024—Anatomic and clinical pathology reporting—what’s working, what’s missing. Three pathologists (all board certified in informatics) and representatives of three information system companies met online Dec. 19 with CAP TODAY publisher Bob McGonnagle to talk about reporting needs, the changes, what’s optimal. The first half of their discussion begins here; the second half will be published in the March issue.
Read More »More progress, fewer barriers for PGx testing
January 2024—Sometimes even superb ideas can also turn out to be quite, well, bothersome. Zoom meetings. Bridal showers. Bike lanes. Parking apps. QR menu codes. And—if laboratories aren’t careful—the same can be true of pharmacogenomic testing. Just ask Ann Moyer, MD, PhD, associate professor, laboratory medicine and pathology, Mayo Clinic. When it comes to pharmacogenomic testing, laboratory medicine brings significant expertise to the table. But in clinical settings, physicians who prescribe the medications need to be familiar with how to use the test results. They also need to work with the lab to decide which tests, for which genes or gene-drug pairs, will be most helpful for their patients, she says. “Especially if you’re going to start incorporating clinical support alerts into the EHR,” adds Dr. Moyer, who was chair of (until Dec. 31; she is now advisor to) the CAP/ACMG Biochemical and Molecular Genetics Committee. “If the practice doesn’t actually want them, then you’re just going to end up annoying them.”
Read More »The who, when, and why of thrombophilia testing
January 2024—Thrombophilia testing has been shown to be performed far more often than indicated in thromboembolic events, at significant cost to the patient and hospital.
Read More »Lab-developed test proposal reflections and predictions
January 2024—The Food and Drug Administration’s proposed rule on laboratory-developed tests would phase out its existing enforcement discretion approach for oversight of LDTs. Instead, the FDA would classify in vitro diagnostics offered as LDTs as class I, II, or III medical devices depending on their risk to patients.
Read More »A scan of studies on HER2-low breast cancer scoring
January 2024—Much has been said and written about scoring HER2-low breast cancer, and it has its difficulties. But there are steps and tools to support scoring, and Savitri Krishnamurthy, MD, last fall shined a light on them and several HER2-low breast cancer-related studies.
Read More »7 pointers for POC cardiac troponin measurement
January 2024—Seven recommendations for the use of cardiac troponin measurement at the point of care were published last year and reported in a session at the Association for Diagnostics and Laboratory Medicine annual meeting, shortly after the recommendations appeared in print.
Thoracic SMARCA4-deficient undifferentiated tumor
January 2024—Thoracic SMARCA4-deficient undifferentiated tumor (TSDUT), formerly known as SMARCA4-deficient thoracic sarcoma and SMARCA4-deficient thoracic sarcomatoid tumor, is a relatively newly defined entity with a distinct clinical history, morphology, immunohistochemical profile, molecular findings, and clinical behavior.
Read More »Bethesda System for Reporting Thyroid Cytopathology
January 2024—The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) is a widely endorsed and globally adapted standardized reporting system of thyroid fine-needle aspiration specimens.
Read More »Reporting urine cytology: how Paris 2.0 differs from 1.0
January 2024—Urinary cytology is widely used to screen for high-grade urothelial carcinoma (HGUC) and to monitor for recurrence. Several reporting systems have been proposed over the past few decades, but The Paris System (TPS) for Reporting Urinary Cytology is the most widely applied worldwide.
Read More »Panelists on viscoelastic and other coag assays
January 2024—Viscoelastic assays and other coagulation tests were front and center when CAP TODAY publisher Bob McGonnagle on Nov. 20 convened seven people in an online roundtable. Oksana Volod, MD, and Eric Salazar, MD, PhD, and five company representatives weighed in on, among other things, appropriate test use, automation, and laboratory-developed tests. What they said begins here.
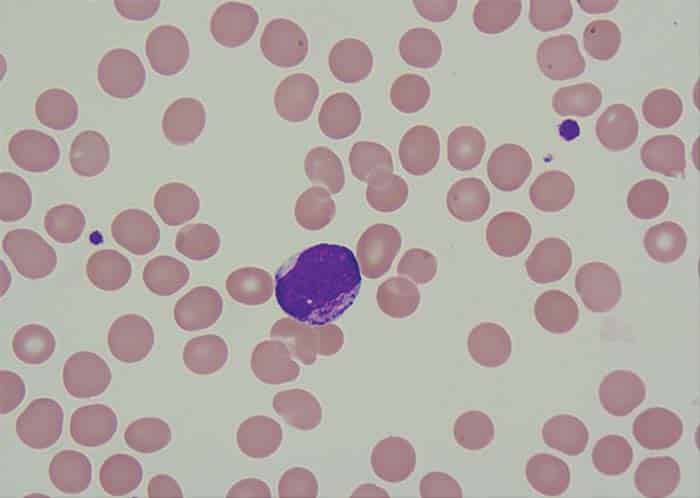
Read More »And the band neutrophil counts play on
December 2023—The recent CAP proficiency testing questionnaire was meant to be the coup de grâce. Hematology PT participants were asked about their band neutrophil reporting practices and, given that these manually generated counts were supposedly on their way out decades ago, the authors of the survey questionnaire expected to see very little activity. The survey, they hoped, would be a way to pound the final, data-driven nail in the coffin. Or, as lead author Maria (Ria) Vergara-Lluri, MD, puts it, “We thought this had all been laid to rest 30 years ago.” It wasn’t. Says Dr. Vergara-Lluri: “Surprise: 86 percent of labs that participated still report bands.” The results of the survey upended many of the assumptions, if not hopes, the authors might have had. Among laboratories that reported manual differentials, they found that most reported bands (4,554 of 5,268). Moreover, only 73 percent reported band reference ranges. On the morphologic challenge, bands classified as “easy” were indeed easy—participants classified them well.
Read More »In fee schedule final rule, lower cuts than proposed
December 2023—In the 2024 Medicare physician fee schedule final rule, the Centers for Medicare and Medicaid Services reacted favorably to the CAP’s advocacy to mitigate payment decreases to pathologists next year. Overall, payments to pathologists are expected to decrease by an estimated 2.7 percent.
Read More »New guidance on lab analysis in diabetes
December 2023—The third and latest edition of recommendations for laboratory analysis in diagnosing and managing diabetes mellitus, released this summer, provide guidance on, among other things, ketone testing, glycolysis, and point-of-care testing. The last such recommendations were published in 2011.
Read More »In ED/urgent cares, the lab tests and the POC team
December 2023—A point-of-care testing team from TriCore was part of standing up three dual emergency department/urgent care centers in as many years, with a fourth set to open in March 2024. “They are super busy, as was expected. There’s a great need for this type of site,” says Kathleen David, MT(ASCP).
Study of suspected transfusion reactions to begin Jan. 1
A new CAP Quality Practices study on rates and turnaround times for investigating and reporting suspected transfusion reactions will begin on Jan. 1, 2024. Enrollment is open now and will continue through Feb. 5, 2024.
Read More »Digital pathology and AI—drivers, budgets, and jobs
December 2023—Digital pathology and AI—the push, the potential, the changing questions, the reimbursement, and the caution. All that and more came up when CAP TODAY publisher Bob McGonnagle on Oct. 17 led a conversation online with pathologists and industry representatives.
Read More »Savings follow allergy, autoimmune test consolidation
December 2023—Hoi-Ying Elsie Yu, PhD, D(ABCC), isn’t new to workflow optimization. As system director of chemistry, point-of-care testing, and preanalytics for Geisinger Medical Center in Danville, Pa., for the past decade, she has undertaken initiatives to maximize efficiency in complicated parts of the laboratory whenever she can.
Read More »Phlebotomy program gives lift to lab, community
December 2023—The clinical laboratory at Children’s Hospital of Philadelphia is solving two problems at once: its phlebotomist staffing shortage and the need for some in its community to learn a new skill and obtain employment.
Read More »LDT proposal on the radar—little detail, clarity needed
December 2023—For most, laboratory staffing woes continue, despite some letup post-pandemic. CAP TODAY publisher Bob McGonnagle on Nov. 7 got a sampling of where staffing stands as the year end approaches, in his conversation online with members of the Compass Group, an organization of not-for-profit IDN system lab leaders who collaborate to identify and share best practices and strategies. But first a few words from them about the Food and Drug Administration’s proposed rule on laboratory-developed tests.
Read More »AMP case report: Potential von Hippel-Lindau syndrome in a patient with negative germline testing
CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Washington University School of Medicine in St. Louis. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »In memoriam
John Kelly Duckworth, MD 1928–2023 December 2023—John Kelly Duckworth, MD, a member of the CAP Board of Governors from 1987 to 1993, died on Sept. 14 at age 95. Dr. Duckworth was the second chair of the CAP Commission on Laboratory Accreditation. He also was chair of the Council on Practice Management, vice chair of the Council on Scientific Affairs, ...
Read More »Test adds twists to lung disease diagnosis
November 2023—It was a mystery, wrapped less in an enigma than a few layers of bafflement, surprise, and mild irritation. Call it the Case of the Split Lung Specimens. The first hint something was amiss came when Alain Borczuk, MD, vice chair of anatomic pathology and co-director of thoracic pathology, Northwell Health, noticed that he and his colleagues were receiving more insufficient bronchoscopy specimens than usual. “When I say ‘increasing’—we don’t get that many bronchoscopies. It’s not like colon polyps,” says Dr. Borczuk, who is also director of oncologic pathology, Northwell Health Cancer Institute. Normally they would get a handful a week, some of them straightforward cancer cases, although these additional cases were tied to noncancerous conditions. And then the plot thickened even further, with missing pieces—literally. Though no guideline clearly states what constitutes an adequate specimen, Dr. Borczuk says, the samples he and his colleagues were seeing fell markedly short.
Read More »New guidance in checklist on AMR and mass spec
November 2023—In the 2023 edition of the CAP accreditation program checklists is new guidance on analytical measurement range verification and new and revised requirements for mass spectrometry.
Read More »Point-of-care testing scorecard spotlights hits and misses
November 2023—At the point of care, there are testing wins, some losses, and plenty of pitfalls. “Point-of-care coordinators all have the same problems,” says Meaghan Gladstone, applications consultant at Werfen.
In some settings, alternatives to HbA1c acceptable
November 2023—Glycated albumin and fructosamine are highly specific, with high levels suggesting hyperglycemia. This points to their utility in monitoring glycemic control in people with diabetes. “They’re quite useful in the setting of overt hyperglycemia,” said Elizabeth Selvin, PhD, MPH, at this year’s meeting of the Association for Diagnostics and Laboratory Medicine.
Read More »Generative AI, from education to corner cases
Generative artificial intelligence—what it is, how it can be used in pathology, what stands in its way, why the excitement. CAP TODAY publisher Bob McGonnagle spoke about that and more with pathologists Bobbi Pritt, MD, MSc, and Scott Anderson, MD; Ajit Singh, PhD, of Stanford and Artiman Ventures; and Devon Snedden, a health care consultant in artificial intelligence. “There are a lot of excellent possibilities that we’re just starting to understand and explore for the field of pathology,” said Dr. Pritt of Mayo Clinic.
Read More »AI-driven spatial biology: the next next-gen sequencing
November 2023—Spatial biology may be an emerging field, but Kenneth Bloom, MD, says he and other pathologists have been doing it “since we got the microscope.” And he argues it’s going to become “the new, most important lens we look through.” The reason is the emergence of new cancer treatments like immunotherapy and, most importantly, antibody drug conjugates like Enhertu, says Dr. Bloom, head of pathology for Nucleai, a company specializing in AI-powered spatial biology.
Read More »Minds shift on digital path, ‘massive change’ predicted
Is digital pathology on the move? Two who know it well say it is. Esther Abels, a precision medicine and biomedical regulatory health science expert who is CEO of SolarisRTC and former president of the Digital Pathology Association, and Michael Rivers, vice president/lifecycle leader of digital pathology at Roche Tissue Diagnostics, spoke in September with CAP TODAY publisher Bob McGonnagle, who got their take on where things stand.
Read More »Pathology navigators bring molecular test efficiencies
November 2023—Few things in the laboratory can do so much at once: boost histotechnologist productivity, safeguard tissue, offer a career path and help retain staff, keep watch on test utilization, and reduce the number of calls to pathologists and turnaround time, all while advocating for the patient.
Read More »Reports revisited—panel on preferences and pain points
November 2023—Reports—integrated or otherwise—were up for discussion when CAP TODAY publisher Bob McGonnagle convened online in October a group of informatics experts, who spoke of the need for simplicity in a time of growing complexity, ease of access, where Epic isn’t strong. The full conversation follows.
Read More »Digital path’s star rises from the mists
October 2023—In living up to its promise as a new technology that will revolutionize clinical care through greater ease, speed, and accuracy of diagnosis, digital pathology has been sluggish. While many analysts, starting at least two decades ago, forecasted that digital pathology would elbow aside glass slides for good, that milestone is still far out of reach. As health economist and chief executive officer of the New York City-based digital pathology company Paige, Andy Moye, PhD, puts it bluntly: “In probably 90 to 95 percent of the cases in the U.S., a pathologist still makes the diagnosis of cancer the way they did it back in 1910: by looking at a glass slide under a microscope.” Mark Lloyd, PhD, vice president of pathology for Fujifilm, says he wouldn’t be surprised to hear that perhaps only five percent to 10 percent of hospitals have moved beyond using only glass slides to offer pathologists digital pathology capability. In fact, Dr. Lloyd thinks those percentages are overstated. What is the market share for the clinical use of digital pathology?
Read More »Looking ahead to respiratory virus season
October 2023—With respiratory virus season near, those with a close eye on it in August gave the lay of the land for test algorithms, technologies, and forecasts, even as SARS-CoV-2 and RSV cases were rising in parts of the country.
Read More »Soon to be required: current susceptibility testing breakpoints
October 2023—A CAP accreditation program requirement that microbiology laboratories use current antimicrobial susceptibility testing breakpoints, which was added to the checklist in 2021, will go into effect Jan. 1, 2024.
Read More »Inside lab’s experience with assays for Alzheimer’s
October 2023—Orders for cerebrospinal fluid testing for Alzheimer’s disease have grown at Mayo Clinic since spring 2020, when testing was first offered. When aducanumab was approved in May 2021, test orders jumped.
Read More »Fast or comprehensive? Lab offers both for NSCLC
October 2023—For molecular testing in oncology, the choice is often fast or slow. PCR-based platforms are rapid, and comprehensive genomic profiling by next-generation sequencing is slower, and each has its pros and cons.
Read More »New guide to whole blood viscoelastic assays: hemostasis, testing, cases, and applications
October 2023—New this month from CAP Publications is Whole Blood Viscoelastic Assays in Clinical Diagnosis: An Illustrated Case-Based Guide. Viscoelastic testing was designed to determine the cause of intraoperative or trauma-related bleeding to guide hemostatic therapy. CAP TODAY asked the book’s editor, Oksana Volod, MD, about the guide. See her answers and a sample chapter. Dr. Volod is professor of pathology and director of the coagulation consultative service, Cedars-Sinai Medical Center, Los Angeles.
Read More »AMP case report: Identification of multiple germline cancer predisposing gene variants in a single patient during tumor sequencing analysis
October 2023—Next-generation sequencing of tumor tissue has important implications in solid and hematologic malignancies because it can identify genomic variants that provide diagnostic, prognostic, and predictive information to guide clinical management. Variants identified on tumor sequencing can be classified as somatic (acquired after conception) or inherited through germline.
Read More »In hematology, making the most of automated solutions
October 2023—Hematology analyzers and the related workflow, expertise, efficiency, and IT matters were the topic of a roundtable when CAP TODAY publisher Bob McGonnagle met online Aug. 29 with two pathologists and representatives from Horiba, Siemens, Sysmex, CellaVision, Sight, and Abbott. Their conversation follows.
Fernando Chaves, what are the advances in artificial intelligence in the field of hematology, particularly automated hematology, since we spoke during our roundtable at this time last year?
Fernando Chaves, MD, global head of hematology, Siemens Healthineers: Technology now enables full-field digital morphology, a full image of the entire slide scan. Now we can do with hematology what has been done for over a decade in surgical pathology.
In memoriam
October 2023—Thomas Dermott Trainer, MD, a member of the CAP Board of Governors from 1994 to 2000 and secretary-treasurer from 1999 to 2000, died on June 10 at age 94.
Read More »Tests for paraneoplastic syndromes in neurology
September 2023—Laboratory testing for paraneoplastic neurologic syndromes is neither commonplace nor cheap. It also comes with its own enigmatical math, as Michael Levy, MD, PhD, recently experienced. As the director of the Neuroimmunology Clinic and Research Laboratory in Massachusetts General Hospital’s Department of Neurology, Dr. Levy keeps an eye on PNS laboratory testing (which is performed at Mayo Clinic) at his institution.
Many knots to untangle in lab test names
September 2023—Ambiguities, inconsistencies, omissions, and other defects in the naming of laboratory tests can send test orders and results interpretation awry, particularly with some of the most common tests. Even among clinicians and laboratorians working at the same hospital for years, smooth sailing is not guaranteed. The authors of a study published in Archives of Pathology & Laboratory Medicine hope to change that. Their aim is to alert patient-facing providers and laboratories to the risks that ambiguous or nonstandardized laboratory test naming poses and to provide practical rules for minimizing those risks.
For infectious disease, what tests and at what time in disease course
September 2023—For the tickborne and mosquito-borne arboviruses, which are frequently more transient in blood and tissue than other pathogens, both molecular and serologic testing have a place in the diagnostic toolkit.
Read More »Lab’s steps to fewer contaminated urine cultures
September 2023—A casual comment made in a routine exchange in an Avera McKennan Hospital laboratory sparked a five-year campaign to bring down the urine culture contamination rate. “I feel like all I do is report contaminated cultures,” a microbiology technologist said in 2016.
Read More »New viscoelastic testing requirement in checklist
September 2023—A proficiency testing accreditation requirement in the new checklist edition was revised to add clarification, and a new requirement on viscoelastic testing will close an existing gap.
Read More »People, partners, and platforms at the point of care
September 2023—Point-of-care testing—the requests and the committees that oversee them, the connectivity, what AI might bring. CAP TODAY publisher Bob McGonnagle on July 21 met online with a laboratory operations director and a medical director from large health systems and with company representatives for a look at where things stand today. Their conversation follows.
Disruptive technologies—what impact on lab workflow?
September 2023—New from CAP Publications is Disruptive Technologies in Clinical Medicine, by Frederick Kiechle, MD, PhD. In his new book Dr. Kiechle says “disruptive technologies offer new paradigms in diagnostic medicine.” Technology-driven disruptions are stimulated by the need to improve patient care, he writes, and they have been “a feature of the practice of clinical pathology since the inception of the first clinical laboratory in 1895 at the University of Pennsylvania, the William Pepper Laboratory.”
Read More »AMP case report: Lung micropapillary adenocarcinomas revisited
September 2023—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Henry Ford Hospital. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »Genetic counseling within the laboratory: For oncology cases, lab’s consult service plugs gap
September 2023—What happens when an oncologist cannot confidently determine what type of genetic test to order for their patient? Where can a provider turn if they do not know whether a genetic variant is clinically actionable? As genetic testing becomes a more integral part of personalized medicine and health care in general, there is a growing need to bridge the gap between those skilled in molecular diagnostics and those on the patient-facing side of care. In response to this need, the Center for Integrated Diagnostics (CID), a high-complexity molecular diagnostics laboratory at Massachusetts General Hospital, created its Consultation Service.
Read More »Low level limbo in HER2 breast cancer
August 2023—Seemingly channeling the inspiration of Magritte and his famous pipe, pathologists are painting a new picture of what has long been an everyday object in their own world: HER2. To paraphrase the master: Ceci n’est pas facile. For years, HER2 testing in breast cancer has seemed self-evident, ever since the HER2-targeted therapy trastuzumab and its companion diagnostic arrived on the scene a quarter of a century ago. Pathologists became comfortable using immunohistochemistry to identify 3+ cases and turning to in situ hybridization techniques to sort through less obvious ones. But early last summer, a variant of the drug, trastuzumab-deruxtecan (T-DXd), shook up that routine. When researchers presented results from the Destiny-Breast04 study at the 2022 ASCO annual meeting, showing that T-DXd significantly improves survival in so-called HER2-low metastatic breast cancer, attendees responded with a minutes-long standing ovation. They then returned from the meeting like evangelicals from the revival tent.
Read More »For patients, demystifying pathology reports
August 2023—With patient access to pathology reports now common via online portals, the question some have asked is what can be done to make them easier for patients to understand.
Read More »Few but notable— new accreditation checklist changes
August 2023—Climate control, calculation verification, block retention, and histocompatibility section director (technical supervisor) qualifications are among the areas in which laboratories can expect to see revisions in the new edition of the CAP laboratory accreditation checklists, to be released this month.
Read More »Upon viral infection, assessing the host nasal epigenome
August 2023—Analyzing the nasal epigenome can shed light on viral infections, strain differences, and potentially infection severity, and for influenza B in particular the results are striking.
Read More »In anatomic pathology labs, a balancing act
August 2023—Anatomic pathology laboratories—the pressures, the promise of technology to alleviate them, and the seemingly unprecedented rates of change. CAP TODAY publisher Bob McGonnagle gathered pathologists and company representatives online on June 21 to talk about it all. From pathologist coverage to IT, from tumor boards to questions job candidates in pathology should ask, here’s what they told us.
Read More »New cassette printer a standout? Users think so
August 2023—No jams, downtime, or lost time. Plenty of savings, speed, and employee satisfaction. Users of what General Data calls its next-generation cassette printer for histology laboratories say they’ve seen all that and more.
Read More »AMP case report: Small intragenic structural variants in SATB2-associated syndrome
CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Washington University School of Medicine in St. Louis.
Read More »Cytopathology in focus: How to approach cytology of unknown primary
August 2023—We discuss in this article a common problem that all cytopathologists come across frequently in their practice: tumors of unknown primary origin involving body fluids and other sites. Metastatic tumor cells can disseminate and colonize discontinuous secondary body sites.1 Such tumor metastases may be the patient’s initial presenting complaint to a family physician for deep-seated tumor primaries such as ovaries, pancreas, liver, and certain non-obstructive gastrointestinal tumors.
Read More »Cytopathology in focus: Impact of primary HPV testing on cytology lab statistical analysis
August 2023—Cytology gynecologic statistical laboratory data must be analyzed in the context of changing screening modalities and patient populations. This is especially important with the increasing use of primary high-risk HPV screening because diagnostic metrics will be markedly different in that patient population.
Read More »Cytopathology in Focus: Lung cytopathology reporting: WHO system and cases
August 2023—Accurate and timely diagnosis is the cornerstone of effective patient care, particularly in the field of pulmonary pathology. To address the challenges health care professionals face in diagnosing and reporting respiratory conditions, the International Academy of Cytology, together with the International Agency for Research on Cancer, recently developed the World Health Organization Reporting System for Lung Cytopathology
Read More »In memoriam
August 2023—Peachy Ridgway Gilmer Jr., MD, a member of the CAP Board of Governors from 1985 to 1991, died on June 18 at age 90. Dr. Gilmer was vice chair of the CAP Council on Education and Membership Services and a member of several CAP groups: the Commission on Laboratory Accreditation, the Council on Quality Assurance, and the Election Oversight, Planning and Priorities, and Surveys committees.
Read More »Turning questions to answers in drug testing
July 2023—As she surveys the opioid epidemic in North America, Christine Snozek, PhD, D(ABCC), could be tempted to think that a ripped-from-the-headlines reality has landed in clinical laboratories as well as on TV crime dramas. With the number of opioid-related deaths increasing in recent years, particularly since the start of the pandemic, drug testing demands have increased for labs as well, says Dr. Snozek, codirector of clinical chemistry and support services and director of point of care and central processing at Mayo Clinic in Arizona. If only she could turn to the entertainment industry for a technology-based solution. “I wish we had CSI lab capabilities,” she says, referring to the long-running police procedural. “You could run a sample and find all the drugs known to man on one test. If they could go ahead and release that technology, that would be wonderful,” jokes Dr. Snozek. Given that laboratories are unlikely to take a meeting with network executives, Dr. Snozek and others in the field will have to look elsewhere.
Read More »For SARS-CoV-2, clearing the air on EUA tests
July 2023—As the COVID-19 federal public health emergency drew to a close in mid-May, industry experts explained what will and won’t change for the laboratory and weighed the fallout from the drop-off in SARS-CoV-2 testing.
The human gut microbiome and blood biochemistry connection
July 2023—The human microbiome has been called the forgotten organ, and at one time it was. But not in the past 10 years. James Versalovic, MD, PhD, made that clear in his talk at the Association for Molecular Pathology meeting last year.
Read More »For sepsis Dx, MDW biomarker brought into the mix
July 2023—When Butler Health System in early 2020 installed the Beckman Coulter DxA 5000 automation line, its hospitals were among the first in the country to do so. At the same time, Butler went live with the DxH 900 hematology analyzer.
Read More »Cytomegalovirus in IBD: where to biopsy, whom to treat
July 2023—Though it’s been suggested that newer drugs have made cytomegalovirus less relevant in patients with inflammatory bowel disease, CMV remains an important opportunistic infection in patients with IBD. Knowing where to biopsy and how many are needed is one of the histologic challenges, said Joseph Misdraji, MD, associate professor of pathology, Yale School of Medicine, in a CAP22 session.
Read More »First a probe purchase, then an academic consortium
July 2023—Bringing new technology into laboratories is important for pathology as a field and for patients—and only getting more difficult. “Each new wave of technology is more complicated than the last,” Jeremy Segal, MD, PhD, said at the USCAP meeting this spring.
Read More »Lab leaders on moving markets and tipping points
July 2023—Digital pathology, the pathology workforce, and the clinical demand for subspecialty expertise were some of what Compass Group lab leaders took on in their June 6 conversation, with CAP TODAY publisher Bob McGonnagle leading the way.
AMP case report: A germline GATA2 c.121C>G (p.P41A) variant in a patient with an unusual acute promyelocytic leukemia
July 2023—A germline GATA2 c.121C>G (p.P41A) variant in a patient with an unusual acute promyelocytic leukemia CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Emory University School of Medicine. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »In search for Candida auris, labs all in
June 2023—A bad-news, good-news, bad-news, good-news bass line thrums through the ongoing story of Candida auris as it continues to spread in the United States. Initially identified in Japan, in 2009, in an ear specimen—hence the auris—the yeast was first reported in the United States in 2016. Like certain other pathogens, C. auris’ domestic presence appeared to be linked to travel-related cases, then quickly spread, first to the metropolitan regions of Chicago and New York City and now to more than half the states. That’s worrisome. Yet the spread hasn’t been unbridled. Early fears that it would sweep indiscriminately through all patient populations have not been realized. “It’s not as virulent as albicans,” says Sixto M. Leal Jr., MD, PhD, director of the clinical microbiology laboratory and of the fungal reference laboratory, University of Alabama at Birmingham, and a member of the CAP Microbiology Committee. “It’s about as virulent as Candida glabrata. It’s not too much of a significant threat if you’re healthy.”
Lyme algorithms: stick to standard, move to modified?
June 2023—For Lyme disease testing, immunoblots became optional in 2019 when the FDA cleared enzyme immunoassays for use as part of a modified two-tiered testing algorithm. “It was a historic event in the world of Lyme diagnostics,” says Elitza Theel, PhD, D(ABMM).
Reorganize, promote, shift, assess—staying staffed amid a shortage
June 2023—The labor shortage may ease now and then for some laboratories in some areas, but the general outlook is that it will stick around for a while—if not forever.
Read More »Weighing the risks in HIV, HCV algorithmic testing
June 2023—For HIV and HCV algorithmic testing, the workflow options have risks to consider. Molecular testing performed as an automatic reflex on the same sample used for the serologic testing risks carryover contamination, and requiring a dedicated sample for the molecular assay risks incomplete testing.
Read More »Workflow, specimen transport under the lens at AACC
June 2023—Transporting specimens to the laboratory, and processing and distributing them within the lab, will be what AACC meeting-goers hear about in a session next month.
Read More »Kaiser, Geisinger: the first of similar headlines?
June 2023—Consolidation in health care makes the news often. But the coming together of Kaiser Permanente and Geisinger Health and their launch of Risant Health got special attention. CAP TODAY publisher Bob McGonnagle asked Compass Group members for their take on the acquisition when they met online on May 2.
Read More »ABPath’s plans for transparency, competency pilot
June 2023—Three initiatives are underway at the American Board of Pathology, one of which its CEO Gary Procop, MD, MS, describes as “a new era of transparency and collaboration.”
Read More »Appendiceal lesions: features, subtypes, patterns
June 2023—Although goblet cell adenocarcinoma can label for neuroendocrine markers, it behaves as an adenocarcinoma and is staged as such. And it’s important to distinguish goblet cell adenocarcinoma from tubular neuroendocrine tumor, a rare subtype of neuroendocrine tumor.
Read More »The intersection of news, core labs, and lab costs
June 2023—As CAP TODAY assembled its annual guides to chemistry and immunoassay analyzers (for this issue and the July issue), publisher Bob McGonnagle brought together IVD manufacturers and lab leaders to talk about consolidation and ever-larger health systems, technology, efficiencies, and centralized and decentralized testing.
Colorectal cancer next on HER2 horizon
May 2023—Behold the common coin. Note its two sides, its easy flippability. Here is Joseph Pizzolato, MD, with the first coin toss. Given the expanded use of biomarkers with a variety of tumors, and constantly evolving assays, how hard is it for medical oncologists to navigate testing? “It’s not difficult at all now,” says a cheerful Dr. Pizzolato, medical director of the comprehensive therapeutic unit of Sylvester Comprehensive Cancer Center, University of Miami Health System, as well as medical director of the Aventura satellite at Sylvester. With third-party companies integrating test ordering directly into electronic medical records, he adds, “It’s getting even easier to order tests and see the results.” Agreed, says his colleague Rhonda Yantiss, MD, director of surgical pathology, Department of Pathology and Laboratory Medicine, University of Miami Miller School of Medicine. And therein lies the problem. “It’s kind of a mess,” she says. In practice, precision medicine is becoming both more and less precise.
Read More »Alzheimer’s biomarkers: CSF reports, plasma prospects
May 2023—For fluid biomarkers in the diagnosis of Alzheimer’s disease, the pace of research and related industry and regulatory developments in the past year has been brisk.
Read More »‘Hundreds of variants’—Staying alert to HbA1c method interference
May 2023—Of the five methods used to measure HbA1c—immunoassay, boronate affinity, enzymatic, capillary electrophoresis, and ion-exchange HPLC—only the latter two can alert the laboratory and physician to the presence of a suspected hemoglobin variant. Even so, there are reasons to be cautious.
Read More »In-house or send-out? Lab approaches to gene panels
May 2023—Achieving standardization and setting up processes around the use of next-generation sequencing panels for the care of patients with cancer is a long road requiring a lot of expertise.
Read More »Supported? Satisfied? Keeping staff from moving on
May 2023—When hiring is difficult, how to improve retention becomes what it’s all about. Linking sign-on bonuses with performance metrics rather than time in the job is one way to try to retain employees in an industry in which demand for staff far outweighs the supply.
Read More »Appendiceal lesion cases, clues, and cautions
May 2023—How to distinguish appendiceal diverticular disease and appendiceal polyps from mucinous neoplasms was just part of a CAP22 course on appendiceal lesions, led by Maryam Pezhouh, MD, MSc, of the University of California, San Diego, and Jacqueline Birkness-Gartman, MD, of Johns Hopkins University School of Medicine.
Read More »The outlook for in-house next-generation sequencing
May 2023—Bringing next-generation sequencing in-house was at the center of a March 27 roundtable led by CAP TODAY publisher Bob McGonnagle, with costs, reimbursement, equity, and the electronic health record part of the conversation. Jeremy Segal, MD, PhD, of the University of Chicago, explains why the Genomics Organization for Academic Laboratories was formed. “By lowering barriers and encouraging cooperation,” he said, “we’ve seen our labs increase the pace of development and the quality of the assays they’re bringing on.”
Read More »Inside the WHO Reporting System for Pancreaticobiliary Cytopathology
May 2023—Standardized reporting systems have been developed during the past decade for cytopathology of different organ systems including the pancreaticobiliary system. The Papanicolaou Society of Cytopathology (PSC) in 2014 published the first reporting system for pancreaticobiliary cytology. Studies have demonstrated that implementation of the PSC reporting system has significantly reduced the number of “atypical” interpretations and increased the number of specific diagnoses.
Read More »Adequacy in cytopathology: focus on cytology specimen use in molecular testing
May 2023—In the first article in our series on adequacy in cytology, published in January 2023 (bit.ly/3MDNVzr), we summarized current approaches to defining adequacy for the purpose of primary diagnosis in the majority of specimen types encountered routinely in cytology practice. As we saw, while the essence of adequacy is constant across reporting systems, the technical definitions can vary significantly by specimen type.
Read More »Growing pains put gene panels in a pinch
April 2023—After years of excitement and scientific breakthroughs, the use of molecular testing to guide cancer therapeutics finally is coming into its own. Unfortunately, it appears to have landed in the wrong place at the right time. That place is a lonely spot, surrounded by gaps in economics and coverage, as well as knowledge, guidelines, ordering patterns, turnaround times, reporting, and the like. So plentiful are the gaps that, put together, they could form a vast, inhospitable space, a veritable Colorado Plateau, with molecular testing as a majestic, enticing but remote rocky pinnacle in the middle. Think Monument Valley. It’s worth the trek. The evidence in support of genomic profiling continues to grow. Simply put, “Patients with the right markers who get the right drugs do better,” says Neal Lindeman, MD, vice chair, laboratory medicine and molecular pathology, Department of Pathology and Laboratory Medicine, Weill Cornell Medicine/New York Presbyterian Hospital. But as numerous studies are showing, the lag in testing is growing as well.
Read More »Sorting out celiac disease with serologic testing
April 2023—Celiac disease incidence is up and the diagnostic rate is low, and it can be years from onset of symptoms to diagnosis. “It’s a long diagnostic odyssey, and so in the laboratory business, we’re all in to help,” says Annette Taylor, MS, PhD, associate vice president at Labcorp where she is strategic director of pharmacogenomics and scientific director of molecular genetics.
Read More »After negative CT for brain injury, a biomarker gap
April 2023—Traumatic brain injury triage in the emergency department is badly in need of biomarkers—and ones that can change practice. “If biomarkers don’t change practice, they’re a waste of time,” said W. Frank Peacock IV, MD, professor of emergency medicine, vice chair of research, and research director, Department of Emergency Medicine, Baylor College of Medicine.
Read More »Traumatic brain injury biomarkers
April 2023—Pradip Datta, PhD, D(ABCC), senior staff technical team leader, Siemens Healthineers, Newark, Del., highlighted the list of traumatic brain injury biomarkers—what they are, what’s available, what’s promising.
Read More »Lab leaders on hires, wages, scanners, and storage
April 2023—How is the demand for biomarker tests linked to new oncology drugs playing out in your health system? It is one of several questions laboratory leaders answered in a March 7 Compass Group call led by Stan Schofield, VP and managing principal of the Compass Group and formerly of NorDx/MaineHealth. That and digital pathology and the cost of storage, staffing and wages, the release of results, and the financial implications of the end of the public health emergency were the topics of the day. The Compass Group is an organization of not-for-profit IDN system laboratory leaders who collaborate to identify and share best practices and strategies.
Ancillary tests in gynecologic pathology—what and why
April 2023—With serous ovarian carcinomas and other gynecologic tumors, it’s molecular testing that increasingly sets the treatment course. “As pathologists, it’s exciting to us,” said Sadia Sayeed, MD, speaking at CAP22. “We’re learning that the immunostains we’re interpreting have greater implications than we ever thought they could.”
Read More »A start at standardizing neoplastic cellularity assessment
April 2023—Molecular testing guidelines say that the neoplastic cell content of each specimen should be assessed, but there are no formal recommendations or guidelines on how to do the assessment.
Read More »In billing, No Surprises and other complexities
April 2023—Another administrative layer and “up in the air” is how lab billing experts describe what the No Surprises Act requires of laboratories and where things stand. When they met online March 3 with CAP TODAY publisher Bob McGonnagle, they talked about this and digital pathology and the problems of no or slow payments. “Compared with five years ago, the number of denials has increased and turnaround time on full payment on a claim has lengthened significantly,” said Tom Scheanwald of APS Medical Billing.
Read More »New paths through hematologic neoplasms
March 2023—Updated classifications for hematologic neoplasms are here. Let the complications continue. As with other specialties, hematopathology has been absorbing advances gleaned from molecular and genetic data. In some cases, this can tilt diagnosis away from primarily immunophenotypic approaches. It might lead to splits in what was formerly a single entity. On occasion, it might suggest further testing options that could be of value to patients now, or possibly at a date down the road. Or it might just leave pathologists and their clinical colleagues peering at a lack of data, knowing they have to make decisions nonetheless. Two groups—the World Health Organization and the International Consensus Classification—have put forth classifications to help physicians sort through the complexities. The WHO published a beta version of the fifth edition of its Classification of Haematolymphoid Tumours in July 2022.
Read More »Case review reveals latest on overtransfusion
March 2023—A retrospective study of patients who received blood transfusions at 15 community hospitals found that just over half of the patient encounters reviewed could have been managed without the transfusion of at least one component type, and 45 percent could have been managed without any transfusion.
Read More »Spotlight on ancillary tests in endometrial cancer
March 2023—With endometrial carcinomas and other gynecologic tumors, molecular testing matters, and not only at the diagnostic stage. “We’re rapidly evolving,” said Leslie M. Randall, MD, MAS, division director of gynecologic oncology, Virginia Commonwealth University Health, speaking at CAP22.
Read More »Putting in place a molecular panel for pneumonia
March 2023—When it comes to molecular syndromic panels for pneumonia, there’s both upside and downside, says Neil W. Anderson, MD, D(ABMM), associate professor, Department of Pathology and Laboratory Medicine, Washington University School of Medicine in St. Louis.
Read More »Digging deep to drive recruitment and retention
March 2023—All in on staff retention and solving staff shortages, some made worse temporarily by weather. That’s where laboratories were when Compass Group members told CAP TODAY publisher Bob McGonnagle in their Feb. 7 call where hospitals and labs were aiming their efforts. From safety huddles and stay interviews to arranging for overnight stays and incentives, the work to remain sufficiently staffed continues. The Compass Group is an organization of not-for-profit IDN system laboratory leaders who collaborate to identify and share best practices and strategies.
Read More »For those who want it easy, blood draws anywhere
March 2023—The need was always there for some. For others, it’s a matter of convenience. “Home phlebotomy as a concierge service” is how Michael Eller describes what went live in 2019 in New York at Northwell Health, where he is assistant vice president of business development for its laboratories. “It was just a matter of using the capacity we already had in the field and hiring appropriately as needed,” he says.
Read More »Close-up on AI-driven assistive tools in pathology
March 2023—Assessing cardiac allograft rejection from endomyocardial biopsy and assigning a differential diagnosis to cancers of unknown origin have been shown to get a boost from AI-driven computational pathology models. So too has identifying subregions of high diagnostic value on whole slide images.
Read More »In urinalysis, reflex algorithms and other efficiencies
March 2023—Urinalysis was at the heart of a Feb. 7 discussion between CAP TODAY publisher Bob McGonnagle; Ron Jackups Jr., MD, PhD, of Washington University School of Medicine; and Jason Anderson of Sysmex America. “There’s a lot of room to explore what the optimal parameters are to use with the best specificity and sensitivity for a reflex to the sediment analysis or the culture,” Anderson said. Here’s what he and Dr. Jackups said about reflex testing, automation, and middleware.
Read More »Breast cancer biomarkers, classic and new
February 2023—Like a thriving expat, Deborah Dillon, MD, is comfortable moving within worlds both old and new. Specifically, as a breast and molecular pathologist at Brigham and Women’s Hospital, she appreciates the biomarkers she and her colleagues grew up with, so to speak, as well as those that are part of a more recently arrived-at scenery. Not everyone finds both worlds equally riveting. “A lot of people are much more interested in, and excited by, new markers,” she says. “When I talk to people from pharma, this is what they want to hear about.” So do many pathologists, oncologists, and patients—new markers and new therapies have a way of updating hopes. Dr. Dillon understands the persistent thrill of the new, why people want her to talk the language of PIK3CA, PARP inhibitors, MMR, NTRK fusions, ESR1, and the like. But as an in-demand speaker as well as in a recent interview with CAP TODAY, she also advocates for making the old—the longstanding trinity of ER, PR, and HER2—seem new again.
Read More »Getting syphilis testing right as case rates rise
February 2023—Traditional algorithm? Or reverse? Elitza Theel, PhD, D(ABMM), of Mayo Clinic, in an AACC session last year walked through the two primary diagnostic algorithms for syphilis, explaining where the complexities lie and how her laboratory uncovered inappropriate testing for neurosyphilis.
Read More »In surgical services, stewardship steps reduce lab orders
February 2023—Consensus on the best ways to stem unnecessary laboratory testing, and spare health care systems and patients its negative effects, is still elusive.
Read More »Views on point of care versus core and more
February 2023—Point of care or core lab? An old question but a new conversation, this one on Jan. 12 between Stan Schofield, Brian Durkin, and CAP TODAY publisher Bob McGonnagle (asking the questions). Here’s what they said about that and health care economics, autoimmune testing, tube supplies—and, of course, the labor shortage because it affects nearly everything in health care.
Read More »Too high or low? Too long? Lessons in ergonomics
February 2023—Workspace design seldom supports the tasks workers perform, and pain and productivity loss can be the outcomes. Eliminating the risks at the design stage is therefore the best approach. When that’s not possible, retrofitting to reduce or eliminate some (but not all) of the hazards is an option, though it’s difficult and can be costly, says Marissa Pentico, MS, OT/L, CPE, ergonomics coordinator in the Duke University Occupational and Environmental Safety Office.
Read More »Study: Cardiac biomarkers in transgender people
February 2023—Sex hormones, rather than sex assigned at birth, may be a stronger driver of the observed concentration differences between healthy men and women for biomarkers of cardiac disease, say the authors of a study published in JAMA Cardiology (Greene DN, et al. JAMA Cardiol. 2022;7[11]:1170–1174).
Read More »‘Doing more for less and with less’: Turning to IT
February 2023—As this year’s guide to anatomic pathology computer systems was taking shape, CAP TODAY publisher Bob McGonnagle met online with representatives of five companies and with John Sinard, MD, PhD, of Yale University School of Medicine. They talked about the cloud, CPT codes, training of pathology informaticians, and artificial intelligence, for which the time frame in pathology is far longer than it’s been portrayed, in Dr. Sinard’s view. “It will start to impact the careers of some of our trainees, but it’s probably a 10- to 20-year time frame before it plays a major role,” he said. The view of Joe Nollar of Xifin: “Speculation that AI will someday replace pathologists is completely overblown,” though it will help to triage cases and mitigate risk. Their full conversation, which took place Dec. 20, 2022, follows.
Read More »Against all odds, a histology lab in rural Ghana
February 2023—I was fortunate last summer to be part of a team established to build a histology laboratory in rural Ghana. Despite our many challenges, we succeeded in building a laboratory where specimens can now be processed for pathological interpretation. Although many obstacles remain, this laboratory offers great potential to improve the pathology services provided to a large portion of rural Ghana.
Read More »Evaluating post-treatment breast specimens
January 2023—Laura Esserman, MD, MBA, can still recall her Eureka moment. She had just seen a talk on residual cancer burden by pathologist W. Fraser Symmans, MB.ChB, a pioneer in the field. “When I saw Fraser present this,” says Dr. Esserman, director, University of California San Francisco Breast Care Center, “I knew immediately that MRI would work and that residual cancer burden would complement it. MRI was basically a snapshot of RCB over time. I realized that we had to institute RCB—we had to standardize our approach.” Until then, she and her colleagues across the I-SPY trial sites relied on individual pathologist assessment for each case. The pathologic complete response rate, or pCR, hovered at about 34 percent. That insight was soon followed by another. Intrigued by what she heard, Dr. Esserman and her pathologist colleagues from all the I-SPY sites traveled to MD Anderson, where Dr. Symmans helped develop the residual cancer burden system, for training.
Read More »Ins and outs of von Willebrand factor activity assays
January 2023—Guidelines for the diagnosis of von Willebrand disease, published in 2021, have raised questions about which von Willebrand factor activity assay laboratories should use.
Read More »For blood cultures, a lab’s new system and incubation time
January 2023—For the central microbiology laboratory serving Barnes-Jewish and four other hospitals in the St. Louis area, validating and implementing a new blood culture system and moving to a shorter incubation time came at a perfect time: right before the pandemic.
Read More »New starts: rapid-molecular pullback, fentanyl screen
January 2023—Respiratory viruses were up in most states when Compass Group members met online Dec. 6 with CAP TODAY publisher Bob McGonnagle, and some were looking to centralize their now decentralized rapid molecular testing. At least one system had already done so. In California, a new law requires fentanyl screening be included in drug screens in all general acute-care hospital lab settings.
Read More »Array of flow cytometry cases in new color atlas
January 2023—Due out this spring is the CAP’s Color Atlas of Flow Cytometry. It consists of 71 cases and provides examples of the full range of hematolymphoid diseases that can be productively analyzed by flow cytometric immunophenotyping. Its editors are David Dorfman, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital; William Karlon, MD, PhD, of the University of California San Francisco Medical Center; and Michael Linden, MD, PhD, of M Health Fairview-University of Minnesota Medical Center. CAP TODAY recently asked Dr. Dorfman a few questions about the atlas. His answers to our questions and a sample case follow.
Read More »Cytopathology in Focus: Adequacy in cytopathology: an overview with a focus on FNA of lymph nodes and mass lesions
January 2023—The definition of “adequate” per the Merriam-Webster dictionary is “sufficient for a specific need.” In cytopathology, it is defined by the quantity and quality of the cellular material sampled. The final interpretation of a cytopathology report is almost universally preceded by an adequacy statement. While the essence of “adequacy” stays the same, its application varies depending on the specimen type and the site sampled. Furthermore, in the current era of personalized medicine, the definition of adequacy has expanded from “enough cells to make a morphologic diagnosis” to “enough cells to make a diagnosis and perform ancillary studies.”
Read More »Cytopathology in Focus: Serous fluid cytopathology—recent progress and Yale’s experience
January 2023—In recent years, a standardized classification system for the cytology of serous body cavities has been proposed. The system, known as the International System for Reporting Serous Fluid Cytopathology (ISRSFC), published by Ashish Chandra, et al.,1 in 2020, aims to enhance the reproducibility of cytologic diagnoses, thereby facilitating clearer communication with clinicians. The diagnostic categories are as follows: I. Non-Diagnostic; II. Negative for Malignancy; III. Atypia of Undetermined Significance; IV. Suspicious for Malignancy; V. Malignant. Each progressive diagnostic category from I through V carries with it an increasing risk of malignancy. This brief review article aims to highlight the salient points of each diagnostic category and includes discussion of recent publications and our own institutional experience.
Read More »Volume? Space? Automation decisions in coagulation
January 2023—Automation and point-of-care, reflex, and viscoelastic testing were some of what came up when a group spoke with CAP TODAY publisher Bob McGonnagle in late November about hemostasis testing. Also tossed in: Results reporting to the EHR, which “can always be improved,” said Eric Salazar, MD, PhD, of University of Texas Health San Antonio. And D-dimer, one of the pandemic’s “health care heroes,” said Nichole Howard of Diagnostica Stago. Here’s what they said about all that and more.
Read More »Shorts on Standards: Now out: ISO 15189 new edition on quality and competence
January 2023—The fourth edition of the International Organization for Standardization’s ISO 15189, Medical Laboratories—Requirements for Quality and Competence, was published at the end of 2022. This international standard, adopted as an accreditation standard by many countries around the world, applies principles of quality management to the clinical laboratory and has general requirements for competent performance of testing. In the United States, ISO 15189 has been implemented voluntarily by close to 100 laboratories as an adjunct to CLIA ’88 regulations or CAP accreditation.
Read More »The way forward for prehospital transfusion
December 2022—Ask Leonard Weiss, MD, what his favorite part of his schedule is, and he’s quick to answer that it’s the fieldwork: the helicopter and ambulance dispatches he accompanies once or twice a month as associate medical director of emergency medical services at the University of Pittsburgh Medical Center. Dr. Weiss, who is also assistant professor of emergency medicine and assistant medical director of Pittsburgh’s Stat Medevac service, says one of the UPMC emergency services he strongly supports is the prehospital transfusion of blood products. “Until recently, there wasn’t a lot of evidence to deploy its use on the ground as it is in the air, but thanks to extensive use by the military and scientific evidence of the value of prehospital transfusion,” he says, it is more likely to become part of some hospitals’ emergency medicine programs. The 911 ground-based transfusion program at UPMC and city of Pittsburgh EMS began in 2020. As Dr. Weiss and his UPMC colleagues acknowledge, however, myriad complexities come into play.
Read More »Urine test ordering—good and going for better
December 2022—Reflex urine culture algorithms have become common and have been shown to reduce urine culture utilization, but efforts to sharpen clinical decision support continue.
Read More »In fee schedule, an increase to pathology clinical labor rates
December 2022—The Centers for Medicare and Medicaid Services published on Nov. 1 its final 2023 Medicare physician fee schedule with payment rates and new policies set to take effect next year.
Read More »Digital pathology now, and where to from here
Nearly 800 registrants were at the Digital Pathology Association’s Pathology Visions meeting this fall, and 54 companies exhibited. “There was a great vibe at the meeting. People were mingling, collaborative. Digital pathology is picking up,” says DPA president Esther Abels. Her term as president will end this month and Liron Pantanowitz, MD, PhD, MHA, of the University of Michigan, will step in as president on Jan. 1.
Read More »In toxicology, unraveling the unexpected positives
December 2022—In toxicology testing, cross-reactive compounds, incomplete medical records, immunoassay performance, calibrator drift, and human error all play into unexpected positives.
Read More »New for waived-only labs: a custom GEN checklist
December 2022—Laboratories that provide only waived testing will now have a CAP accreditation program laboratory general (GEN) checklist tailored to waived test procedures, beginning with the 2022 checklist edition released in October.
Read More »Artificial intelligence in pathology: the tools, the talk
December 2022—In September, when CAP TODAY publisher Bob McGonnagle met with pathologists and representatives of companies to talk about laboratory information systems (“Lab information systems—where the needs are greatest,” https://bit.ly/LIS_112022), they talked also about artificial intelligence—innovations, clinical impact, how much interest there is. That part of their conversation follows.
Read More »First Japanese hospital lab earns CAP accreditation
December 2022—Atsushi Ohtsu, MD, PhD, director of Japan’s prestigious National Cancer Center Hospital East, reached a groundbreaking decision with his management team in February 2020: They decided to pursue CAP accreditation. While the CAP has accredited more than 40 laboratories in Japan, this was to be a first for a Japanese hospital. And it was: In September 2022, the CAP advised the cancer center (NCCE) of its success in achieving accreditation and congratulated the team for the excellence of its laboratory services.
Read More »Bright prognosis for brain injury biomarkers
November 2022—The lack of tools for assessing traumatic brain injury has long bedeviled physicians. There’s CT. And then? “This has been an unmet medical need for years,” says Ramon Diaz-Arrastia, MD, PhD, the John McCrea Dickson, MD, professor of neurology and director of the Clinical Traumatic Brain Injury Research Center, University of Pennsylvania Perelman School of Medicine. “As many of us know, it’s one of the major barriers that has hindered clinically advanced development of new therapies in TBI. And I think it’s pretty clear that the clinical evaluation alone leaves a lot to be desired.” “I am always frustrated that we have limited tools,” agrees Frederick Korley, MD, PhD, associate professor and associate chair for research in emergency medicine, University of Michigan Medical School, and scientific director, Massey TBI Grand Challenge, Weil Institute, University of Michigan. That’s now on the cusp of changing. Blood-based biomarkers for brain injury may not be bellying up to the bar just yet, but they are starting to raise the bar for how physicians assess TBI.
Read More »Is apolipoprotein B the best measure of CVD risk?
November 2022—The evidence in favor of measuring apolipoprotein B routinely, with other lipid parameters, is now so overwhelming, says cardiologist Allan Sniderman, MD, that he believes it’s unreasonable to deny patients the advantage of apoB. “If evidence is what counts,” he says, “then the care Americans receive should include apoB.”
Read More »Canadian pathology study finds high burnout prevalence
November 2022—Burnout among Canadian pathologists is prevalent, pain related for some, and workload driven for many. “There needs to be more of us,” says Julia Keith, MD, associate professor in the Department of Laboratory Medicine and Pathobiology at the University of Toronto.
Read More »In toxicology, puzzling out the unexpected negative
November 2022—In cases of unexpected negative results in toxicology testing, avoid overinterpretation, know your assays and providers, and don’t put off definitive testing when it’s needed, though it’s not a panacea.
Read More »The who, what, and when of respiratory virus testing
November 2022—In mid-October, flu was picking up, with high levels of activity in Texas, Georgia, the District of Columbia, South Carolina, Tennessee, and New York. Elsewhere, it was still on the lower side, with less known about what was to come but plans in place. And questions, too, about laboratory testing as it relates to SARS-CoV-2, “which is going to be a challenge,” says David Peaper, MD, PhD, D(ABMM), a member of the CAP Microbiology Committee.
Read More »No time to wait: How rapid NGS changed cancer care
November 2022—Rapid next-generation sequencing in a community hospital setting, performed by histotechnologists and interpreted by anatomic pathologists, is possible and paying off, and it “makes the pathologist a much more meaningful part of the precision oncology team,” says Brandon Sheffield, MD, of the Department of Laboratory Medicine, William Osler Health System, Brampton/Etobicoke, Ontario. “It has changed practice at our hospitals,” he says.
Read More »Lab information systems—where the needs are greatest
November 2022—What labs want and need from their lab information systems and what the missing pieces are in interoperability are what pathologists and LIS company reps talked to CAP TODAY publisher Bob McGonnagle about when they met online Sept. 12. “The biggest challenge is with device integration” in molecular testing, said J. Mark Tuthill, MD, of Henry Ford Health System. “We have million-dollar instruments and we’re still programming runs manually. We don’t have HL7 order feeds. We don’t have the ability to get result feeds outbound from those devices.”
The art and science of positive blood cultures
October 2022—It might be possible to tot up, using only the number of toes on an ordinary foot, how many labs are feeling full of vim and vigor these days, open to concepts like creative destruction and get those creative juices flowing and have fun with it—slogans once easily uttered but now tiring to enact. Nevertheless, Margie Morgan, PhD, D(ABMM), would like her colleagues to at least consider the possibility of inspiration in the microbiology laboratory. In particular, Dr. Morgan, medical director of microbiology and professor of pathology and laboratory medicine, Cedars-Sinai Medical Center, Los Angeles, has some thoughts about using a new automated system to facilitate rapid microbial identification from positive blood cultures. The Arc system, from Accelerate Diagnostics, is composed of the Arc module and blood culture kit and concentrates organisms recovered in positive blood cultures for direct testing on MALDI-TOF mass spectrometry. Dr. Morgan and colleagues have been using the system since February.
Read More »Platelet transfusions: safety, cost, and workflow
October 2022—The jury may no longer be out on whether pathogen reduction of platelet units reduces the risk of a septic transfusion reaction enough to replace culturing of platelet units.
Read More »Checklists now made to fit for next-gen sequencing labs
October 2022—As the diagnostic uses for next-generation sequencing have grown, so too has the length of the NGS section of the CAP molecular pathology accreditation program checklist. Now, with the release of the new checklist edition this month, NGS laboratories will find the NGS section in their customized checklists leaner, more relevant, and easier to read.
Read More »Sodium measurement—when the method matters
October 2022—William E. Winter, MD, D(ABCC), is blunt about whether to report a corrected sodium: He would worry if his name were on such a report. “I think you have to be careful about formulas,” he said in his “hot topic” talk at the AACC meeting in July.
Read More »Purchased for the pandemic? Rethinking instrumentation
October 2022—Who’s doing what with instruments purchased at the peak of the pandemic? That and next-generation sequencing are what CAP TODAY publisher Bob McGonnagle asked Compass Group members about when they met virtually on Sept. 6. The Compass Group is an organization of not-for-profit IDN system laboratory leaders who collaborate to identify and share best practices and strategies.
A wait-and-watch season of respiratory viruses
October 2022—Influenza incidence and what it will mean for testing in this respiratory virus season is a wild card, as is how SARS-CoV-2 will evolve. In early September, SARS-CoV-2 prevalence was declining in parts of the United States. “And if you believe in the theory of viral interference,” says Michelle Tabb, PhD, chief scientific officer at DiaSorin Molecular, “it’s leaving the door wide open right now for something else to step in. We’ll see if that’s RSV, or flu A, or if it’s a new COVID variant.”
Read More »Scoring HER2 expression across the full spectrum
October 2022—HER2-low breast cancers are now of greater clinical interest, given Enhertu’s recent approval for use in treating such cancers. How to achieve accurate and reproducible results in scoring HER2-low tumors was at the center of a CAP TODAY webinar on new perspectives on the full spectrum of HER2 expression in breast cancer.
Read More »Enabling ‘the magic’ in hematology—eyes on what labs need
October 2022—New and better solutions for the hematology laboratory. That was at the center of a Sept. 2 virtual roundtable, led by CAP TODAY publisher Bob McGonnagle. With him were Jonathan Galeotti, MD, of the University of North Carolina School of Medicine, and representatives of Sysmex America, Siemens Healthineers, Beckman Coulter, and CellaVision. “It’s a new era in terms of what can happen in hematological data,” said Fernando Chaves, MD, global head of hematology, Siemens Healthineers.
Read More »Highs, lows of tumor mutation burden testing
September 2022—It may not be the oldest story in the world, but in clinical laboratories it’s an oft-told tale: Tumor meets biomarker; drug meets companion diagnostic; both meet FDA approval; clinicians meet with patients offering new hope—and those in the lab are left trying to figure out how to make it all work. That story is playing out again in the realm of measuring tumor mutational burden. In mid-2020 the FDA approved pembrolizumab as a new treatment option in adult and pediatric patients with TMB-high (≥10 mutations/megabase) solid tumors, as determined by the FDA-approved FoundationOne CDx assay. “That doesn’t sound too controversial, right?” says Alain Borczuk, MD, vice chair of anatomic pathology and director of oncologic pathology, Northwell Health Cancer Institute. “It’s not the only way in, but it’s one of the ways in. If you’re arguing for your patient that this is the biomarker that makes them eligible for the drug, then the next questions will be, What was the number? And what was the test?” And it’s off to the races.
Read More »As blood supply tightens, so too does mitigation
September 2022—Picture a performer juggling tenpins while walking a high wire, knowing that a hurricane looms. Add a safety net that could disappear at any time. That’s a sense of what hospital transfusion services experience in maintaining enough blood products to meet patients’ needs.
Read More »BNP and NT-proBNP: how they differ, what it means
September 2022—Which biomarker for heart failure should be used—BNP or NT-proBNP—and does it matter? That’s the question Christopher W. Farnsworth, PhD, set out to answer in his “hot topic” talk (one of a trio) at the AACC meeting in July.
Read More »New data on rapid rule-out using high-sensitivity cTnT
September 2022—A single high-sensitivity cardiac troponin T measurement below the limit of quantitation of 6 ng/L is a safe and rapid method to identify a substantial number of patients at low risk for acute myocardial injury and infarction, say the authors of a recently published study.
Read More »What’s required in ’23 for predictive marker tests
September 2022—Beginning next year, two additional predictive marker tests will require enrollment in proficiency testing (PT), but for any predictive marker using immunohistochemistry or in situ hybridization, only laboratories that perform both staining and interpretation must participate in CAP-accepted PT.
Read More »Need a written policy or procedure? Look for icon
September 2022—A new icon in the accreditation checklist edition to be released next month will make it easier to know if a written policy or procedure is needed for compliance with a requirement, and easier to inspect and be inspected.
Read More »‘Staff love the change’: moving to MLS and why it matters
September 2022—Never a better time than now to switch from the medical technologist job title to medical laboratory scientist. So says John Waugh of Henry Ford Health System, where the job title was formally changed. “Because our people are scientists,” he explains. Waugh and other Compass Group members met online Aug. 2 with CAP TODAY publisher Bob McGonnagle, where they talked about the job title and testing for monkeypox.
Read More »U.S. blood supply steadier but still short
August 2022—Blood is a precious resource and shouldn’t be treated as a commodity. That’s the consensus in the blood banking community, in line with a longstanding conviction that volunteer donations should remain at the blood system’s core. But as the worst of the pandemic appears to have passed, discussion of blood shortages has increasingly drawn on the vocabulary of commerce, and the warnings about the blood supply have been rife with references to supply chain problems that go beyond the need for more donations. Crises in the blood supply are nothing new, and while the health care system strives to stay prepared, the pandemic threw novel commercial and logistical factors into the mix, in some ways jumbling the expected order of a crisis for blood services. Hospitals scrambled to cope with a surge of COVID-19 patients while the spread of infection caused thousands of blood drives to be canceled, so there was a steep drop in supply of blood products, says Pampee Young, MD, PhD, chief medical officer, biomedical services, American Red Cross.
Read More »Autoverification: lessons drawn from a core lab
August 2022—Just as there is scant room in this world for pink “While You Were Out” notepads, paper checks, or the copying skills of Bartleby the Scrivener, laboratories would do well to leave manual result verification in the past.
Read More »A practical approach to borderline melanocytic neoplasms
August 2022—In cases of borderline melanocytic neoplasms, which have overlapping histopathologic features of benign and melanocytic lesions, additional immunohistochemical studies sometimes help to differentiate the two. But a subset of lesions will show overlapping features.
Read More »Looking for lab staff here, there, and overseas
August 2022—Higher wages help to fill open positions, when they can be offered, but in a labor market that’s as tight as ever, they’re often just a start. That’s why many laboratories are casting wider nets, though the hiring solutions tend to be long term.
Read More »Infectious diseases of the gut
August 2022—The atypia in Epstein-Barr virus-positive mucocutaneous ulcers can mimic diffuse large B-cell lymphoma or classical Hodgkin lymphoma, a diagnostic pitfall that can result in overtreatment. And esophageal ulcers in immunocompromised patients should trigger cytomegalovirus immunohistochemistry in addition to GMS and herpes simplex virus-1 and -2 stains.
Close-up on HER2 alterations in advanced NSCLC
August 2022—HER2 is a known oncogenic driver and emerging biomarker in non-small cell lung cancer, and while the therapeutic implication is not yet fully known in NSCLC, “we need to pay attention to it,” said Fred R. Hirsch, MD, PhD, executive director of the Mount Sinai Center for Thoracic Oncology and associate director, Tisch Cancer Institute, in a CAP TODAY webinar sponsored by Daiichi-Sankyo and AstraZeneca.
Read More »Savings, schedules, new automation—labs weighing it all
August 2022—Running into the reality of staffing. Those are the words of a pathologist who said in the most recent Compass Group roundtable that its health system is making a push to obtain and test specimens “as close to home as possible.” Another Compass Group member said planning is underway for the new hospitals his system is going to build, “but we don’t know where staff will come from.” Here is what they and others told CAP TODAY publisher Bob McGonnagle on July 5.
Read More »Cytopathology in focus: Protocol for reporting cervicovaginal cytology specimens
August 2022—The protocol for the reporting of cervicovaginal cytology, the first in a series of CAP cytopathology protocols, became available for use in a synoptic format on June 22. This protocol is a collaborative effort, based on input from past and present members of the CAP Cytopathology Committee and prepared in conjunction with the CAP Pathology Electronic Reporting Committee. It was presented via webinar to the CAP House of Delegates on March 31. A two-week open comment period followed; all comments were reviewed and appropriate changes were incorporated into the protocol.
Read More »Cytopathology in focus: Advances in detection of mesothelioma in cytology pleural fluid specimens
August 2022—The ability to make a definitive diagnosis of mesothelioma on pleural fluid cytology has been questioned and debated for a long time. The 2018 American Society of Clinical Oncology clinical guidelines limit the cytological diagnosis of pleural fluid specimens only as an initial screening test for mesothelioma. Monaco, et al., discuss in their article the appropriate use of ancillary studies (immunohistochemistry and fluorescence in situ hybridization studies) to make a definitive diagnosis of mesothelioma in small tissue samples, which are often processed as cell blocks. The authors recommend a stepwise approach starting with immunohistochemistry for BAP1 and, next, MTAP in cases of atypical mesothelial proliferations where the suspicion for malignant mesothelioma is high.
Read More »Transgender care, in and beyond the lab
July 2022—Gabrielle Winston-McPherson, PhD, could be talking about almost any aspect of laboratory medicine as she recounts how the Henry Ford Health chemistry division, in which she is associate director, has identified a patient care need. She talks about the desire to improve health outcomes. Identifying problems in the preanalytical process. Appropriate test utilization. Putting together a team to develop training material. Assembling data and information prior to implementation. Informatics challenges. And, naturally, the perpetual financial concern of ensuring allocation of limited resources. How else would she—or any other laboratory professional—talk about the lab’s role in transgender health care? In fact, there are many other ways to discuss the topic. “It’s been in the news a lot these days, obviously,” says Matthew Krasowski, MD, PhD, clinical professor and vice chair, clinical pathology and laboratory services, University of Iowa Hospital and Clinics. In fact, there are many other ways to discuss the topic.
Read More »Monoclonal gammopathies: which tests and why
July 2022—Variability in testing for monoclonal immunoglobulin proteins was in part what led to the new guideline on laboratory detection and initial diagnosis of monoclonal gammopathies, published in May.
Read More »Histology lab tips for top-tier whole slide images
July 2022—Good slides in, good images out. Liron Pantanowitz, MD, MHA, explained how to get those good images in a recent webinar on preanalytics quality control in digital pathology, sponsored by Sunquest and made available by the Association for Pathology Informatics.
Read More »Emergency department tests HIV screening strategy
July 2022—Thanks to more than two years’ experience with SARS-CoV-2, perhaps at no point in U.S. history has the general public been as aware of antigen and PCR testing, and the difference between them, as it is now.
Read More »‘A struggle every day’—outpatient center decisions
July 2022—A time of tough choices. A complex dance. This is how Compass Group members on a call with their colleagues, led by CAP TODAY publisher Bob McGonnagle, describe what it’s like to cover outpatient centers amid severe staff shortages. “We are consuming significant resources to get all our locations staffed,” one member says. Another predicts: “We will not be out of this staffing situation for 10 years.” Here is more of what they and others talked about on June 7 as COVID positivity rates were up and monkeypox was in the news. The Compass Group is an organization of not-for-profit IDN system laboratory leaders who collaborate to identify and share best practices and strategies.
Read More »One hospital’s story: Ins and outs of low titer O whole blood use in trauma
July 2022—Myriad questions had to be answered and plans made to put low titer O whole blood in the trauma bay at Thomas Jefferson University Hospital. Julie Katz Karp, MD, associate professor and director of transfusion medicine, reported why, when, and how it was done and where they stand today, in a process she describes as “a never-ending series of hoops.”
Read More »What’s bugging the gut? A team approach
July 2022—Gut pathogens, their histologic features, and a GI pathology and microbiology team approach to diagnosis were the focus of a CAP21 session, “What’s Bugging the Gut?” Maryam Zenali, MD, Alina Iuga, MD, and Christina Wojewoda, MD, presented a series of cases and highlighted the features, the differential diagnoses, and the integrated workups. Three of their cases follow here, with others to be reported in an upcoming issue.
Read More »How close to patients? Cost, quality, competition
July 2022—Point-of-care versus centralized testing, and automation, IT, and staffing. It all came together as industry executives and a laboratory director and a former medical director met with CAP TODAY publisher Bob McGonnagle on May 25, as CAP TODAY’s list of chemistry and immunoassay analyzers was going together. “I don’t worry much about the machines or reagents,” thanks to good-quality practices, said André Valcour, PhD, MBA, DABCC, of Labcorp, who noted the real focus is quality of information and information transfer. Susan Fuhrman, MD, formerly of OhioHealth, said, “We should always give our clinicians as much information as we can accurately produce and our reports should be as clear as we can make them.” And of the staffing crisis: “We have a perfect storm,” she said. Here is more of what they and the others had to say.
Read More »Breast cancer breakthrough sparks HER2 quest
June 2022—The latest advance in breast cancer treatment is a big one—the promising antibody drug conjugate fam-trastuzumab deruxtecan-nxki, or T-DXd (Enhertu). The drug was granted breakthrough therapy designation this spring for patients with HER2-low metastatic breast cancer, and the drug and trial on which the decision was based were the focus of the plenary session at the ASCO annual meeting in early June. “This drug in particular is a variant of a drug we are all very familiar with—Herceptin, or trastuzumab,” says David Rimm, MD, PhD, the Anthony N. Brady professor of pathology, professor of medicine (oncology), director of the translational pathology and Yale pathology tissue services, and director of the physician scientist training program in pathology, Department of Pathology, Yale University School of Medicine. Also familiar: the IHC test to determine eligibility for the drug, a companion diagnostic developed decades ago. But that’s where easy familiarity ends.
Read More »A single pathway for HIV testing and therapy
June 2022—By revealing the value of a diagnostic algorithm using quantitative RNA as the second test to confirm reactive HIV screening results, Daniel Gromer, MD, and colleagues say their simulation modeling suggests clinical improvement over the standard-of-care algorithm, and at lower cost if HIV specimen positivity is high.
Read More »The post-sophomore fellowship and other pieces of the pipeline
June 2022—Jobs in pathology are plentiful and positions are hard to fill, which has put the pipeline in the limelight. CAP TODAY publisher Bob McGonnagle led a virtual discussion with two pathologists and a pathology resident.
Read More »Lining up for low titer O whole blood in trauma care
June 2022—For many blood suppliers, there is more enthusiasm for low titer O whole blood than there is an ability to make it, especially with the pandemic having made it harder than ever to collect.
Read More »Fluid cytology—key features and ancillary testing
June 2022—What to look for in serous fluid cytology is what Eva M. Wojcik, MD, of Loyola University in Chicago, and Xiaoyin “Sara” Jiang, MD, of Duke Health, set forth in their CAP21 session last year.
Read More »AACC session to zero in on cannabis and driving
June 2022—The effects of acute cannabis on driving performance and how impaired drivers can be detected will be reported at the AACC annual meeting July 24–28. Sessions on serum COVID-19 antibodies and lab workforce solutions are two more of the many that await attendees in Chicago.
Read More »Compass on ‘consumerizing health care’ and more
June 2022—What stood out among all that was seen and heard at the Executive War College? Compass Group members who were there answer CAP TODAY publisher Bob McGonnagle’s question in their early May virtual get-together, shortly after the War College took place. Here’s what they and other lab leaders said about retail lab testing, digital pathology and artificial intelligence, and their plans for the future.
Read More »Race in medicine: Is it data or distraction?
June 2022—How race shows up in the medical school curriculum and what to do about it was the focus of a grand rounds by Andrea T. Deyrup, MD, PhD, and Joseph L. Graves Jr., PhD, presented virtually this spring to the University of Minnesota Department of Laboratory Medicine and Pathology, on behalf of the department’s diversity, equity, and inclusion committee.
Read More »Letters
June 2022—The CAP issued a statement on April 5 in support of the ADVANCE study, which hopes to end discriminatory practices based on sexual identity and/or sexual orientation in donor risk assessments and deferral periods for blood donation. Originally, the FDA instituted such discriminatory criteria on the assumption that potential donors who are gay and bisexual men who have sex with men are at an increased risk of transmission of communicable diseases, such as human immunodeficiency virus. If the results of the ADVANCE study prove this antiquated idea false, the FDA will hopefully amend the official guidance on donor eligibility for blood donation.
Read More »Leaving behind outdated AST breakpoints
May 2022—Among the countless interruptions COVID-19 has inflicted on the medical community, one of the most obvious has been conversational. In the face of a global pandemic, other topics can seem unworthy of discussion. But as some post-pandemic normalcy creeps back in, so does the focus on topics of equal, if less dramatic, importance.
On the track of new approaches to myocarditis
May 2022—Studies show promise for new approaches to biomarkers for myocarditis diagnosis, one of which is circulating microRNA mmu-miR-721. Another biomarker, sera soluble ST2 (sST2), which has been found to be clinically useful in predicting heart failure, could be added to existing biomarkers used to diagnose patients with myocarditis, interpreted according to sex and age. And serial high-sensitivity troponin measurements might be another approach to diagnosing and monitoring myocarditis.
Integrating NGS into the cytopenia workup
May 2022—Myelodysplastic syndromes are often challenging to diagnose, and it’s the exceptions to the rules that make it so, said Phillipp W. Raess, MD, PhD, associate professor of pathology and laboratory medicine, Oregon Health and Science University, speaking at CAP21.
Read More »High hopes for schools as lab positions go unfilled
May 2022—Opening and expanding schools—the path to a labor pool for labs. Compass Group members continue to move on that as they experiment with other solutions. “We’re exploring every avenue to bridge the staffing gap,” said Dhobie Wong of Sutter Health in a virtual roundtable with CAP TODAY publisher Bob McGonnagle on April 5.
Read More »When surgical pathology is key to infectious disease
May 2022—Infectious disease diagnosis sometimes requires a surgical pathologist, often in unexpected situations. In a CAP21 session, “Uncultured: Infectious Diseases in Surgical Pathology,” Sarah D. Hackman, MD, assistant professor, Department of Pathology and Laboratory Medicine, University of Texas Health San Antonio, presented a sampling of such cases, two of which follow.
For inspectors, a new and better training course
May 2022—Users of the CAP’s redesigned laboratory inspector training course, introduced last December, should find it to be more fun, less chore, and tailored to what they need to know, say those who developed the new course. And it’s open and accessible to all.
Read More »In next-gen sequencing, aiming for wider access
May 2022—Next-generation sequencing—the worries, the wins, and what’s new. That’s what came up when CAP TODAY publisher Bob McGonnagle led an NGS-focused roundtable on March 14. With him were Jeremy Segal, MD, PhD, of the University of Chicago; Pierre Del Moral, PhD, MBA, and Fiona Nohilly of Illumina; Sohaib Qureshi, PhD, of Thermo Fisher Scientific; and Andy Johnson, DPhil, of Janssen. Here’s what they had to say.
Read More »Cytopathology in focus: ROSE and telecytopathology: a point-of-care test
May 2022—Substantial progress has been made during the past several years in diagnosing and treating various illnesses. Advances in genetic and genomic science; imaging and localization devices; the use of minimally invasive diagnostic sampling procedures; diagnostic, prognostic, and predictive testing; and personalized therapeutic options—all have changed the pattern of the practice of medicine and how patient care is provided.
Read More »Cytopathology in focus: The cytopathology workforce through a DEI lens
May 2022—The ineffectiveness of the U.S. health care system is well documented. The United States consistently allocates more resources for health care compared with other industrialized countries, while not holding the top spots for desired outcomes. A significant percentage of Americans is underinsured or uninsured, and access to quality care is widely asymmetrical among different racial and ethnic groups. Early in the pandemic, COVID-19 highlighted these health inequities in which Blacks, Hispanics, Native Americans, and immigrants were the populations to disproportionately experience disparities related to burden of disease and mortality.
Read More »Cytopathology in focus: Know the accreditation requirements for telecytology
May 2022—The number of minimally invasive fine-needle aspirations requiring rapid on-site evaluation (ROSE) in the cytopathology laboratory has increased over the past decade. Laboratories have seen lower gynecologic volumes and an increase in both nongynecologic fine-needle aspiration biopsy and touch imprint samples. ROSE for patient care has proven value. Sample adequacy allows for a single visit and avoids having to make multiple attempts to provide material sufficient for all required testing, including flow cytometry, microbiology, cell block preparation for immunohistochemical and histochemical staining, and molecular testing.
Read More »Pathology hospitalists in place at UMich
April 2022—Asked why he robs only trains, Richard Farnsworth’s Gentleman Bandit in The Grey Fox answers with a truth universally acknowledged: “A professional always specializes.” In line with that conviction, there’s little debate on the value of specialization in medicine—or, as it has evolved more recently, the extraordinary value of subspecialization in anatomic pathology. Many consider subspecialist signout to be the gold standard of review and diagnosis in pathology. Because they are dealing with a small number of pathologies, “the care that subspecialists can provide is phenomenal,” says L. Priya Kunju, MD, director of surgical pathology at University of Michigan Health. But in hospital practice at academic institutions like the University of Michigan, when it comes to time-sensitive frozen sections, subspecialization can have a downside. The need to return a diagnosis of a frozen section within 20 minutes while a surgery is in progress may require that an array of different subspecialists be close at hand, near the operating room.
Read More »Lab workforce crisis takes top spot
April 2022—There are sudden crises like the SARS-CoV-2 pandemic. And then there are the simmering crises that can be temporarily overshadowed but inevitably re-emerge to command notice. In New York and in many other states, the decades-long shortage of laboratory staff—especially medical technologists and histotechnologists—has gone from simmer to rolling boil.
Read More »First IQCP template set up for molecular tests
April 2022—Drawing on five years of experience and laboratory feedback, a collaborative team has revised the microbiology IQCP templates and created the first template for the quality control of a commercial cartridge-based molecular test system.
Read More »Supply price hikes now as common as shortages
April 2022—Staffing, supply chain, and significant supply price increases. Two continuing problems and one new. When Compass Group members met online March 1 with CAP TODAY publisher Bob McGonnagle, that trio of issues took center stage. Here’s what they had to say.
Read More »Liver pathology: autoimmune hepatitis, PBC, or overlap?
April 2022—Don’t be afraid of livers. Maryam K. Pezhouh, MD, offered that advice in a CAP21 presentation on autoimmune hepatitis, primary biliary cholangitis, and overlap syndrome, part of a session on common queries in liver pathology. “You don’t need to know everything when you’re looking at the liver,” said Dr. Pezhouh, associate clinical professor of pathology at the University of California, San Diego. “But you need to know what your clinician and your patient are asking.”
Read More »Getting paid: policies, pressures, and a power struggle
April 2022—All things billing, revenue, income, and business-related were tossed around when representatives of four billing companies met online Feb. 14 with CAP TODAY publisher Bob McGonnagle. With them were Vachette Pathology founder Mick Raich and Al Lui, MD, of Innovative Pathology Medical Group, Torrance, Calif. The No Surprises Act, pathologist shortage, pharmacies, and SARS-CoV-2 testing post-pandemic were just some of what came up. Artificial intelligence too. “In the next two or three years, the payers are going to use AI to deny claims,” Raich predicts. “They’re going to know which claims are less likely to be appealed when they’re billed.” And that will only make more difficult an already tough situation. In the past year, says Kyle Fetter of Xifin, “there’s been an increase in the use of the CO-252 rejection/denial code.” Chris Condon of APS Medical Billing agrees, saying the job of the carrier “is to figure out ways to not pay pathologists.”
Read More »The impact of diagnostics on antimicrobial decisions
April 2022—A study published last fall examined antimicrobial prescribing in gram-negative bloodstream infections based on three rapid diagnostic panels and using what’s known as the DOOR-MAT framework. The study’s findings were explained in a CAP TODAY webinar on stewardship interventions to optimize the management of gram-negative bacteremia. It was presented last December and made possible by a special educational grant from BioFire.
AMP case report: ETV6/FLT3 fusion gene detected in a patient with T-cell lymphoblastic lymphoma
April 2022—Genetic alterations of the gene FLT3, especially internal tandem duplications in the juxtamembrane domain and point mutations in the tyrosine kinase domain, are commonly seen in patients with newly diagnosed myeloid leukemias. However, chromosome rearrangements involving the FLT3 gene are extremely rare in hematologic malignancies. The FLT3 gene has only a few known partner genes, including the gene ETV6, which encodes a transcriptional repressor. ETV6 has a wide variety of translocation partner genes, several of which are tyrosine kinase genes.
Reaching for breakthroughs on burnout
March 2022—Few people want to talk about burnout in health care—at least not publicly. Take, for example, the response of one laboratory professional, who, when asked to be interviewed for this story, waited several days before ultimately declining. Having his institution associated with the topic, he explained, could fan the flames among colleagues. “For sure all of us are feeling weary,” he said in an email (quoted with permission). “And I don’t want this in the face of our team members who are chronically short-staffed while seeing large hiring and retention bonuses going to nurses and others at the bedside. Those payouts are choking off access to capital for replacement equipment and causing every non-nursing position to go through a weekly labor committee review,” with finance leaders evaluating all replacement requests based on funding ability and productivity. “We all have some burnout,” he continued, noting the number of people retiring or trying new careers. The response ticks many boxes on the aspects-of-burnout list.
Read More »Close-up on common diagnoses in core biopsies
March 2022—Papillary and fibroepithelial lesions of the breast were the focus of a talk given by Xiaoxian (Bill) Li, MD, PhD, in a CAP21 session on common but challenging diagnoses in breast core biopsies.
Read More »Dark days are over, but new and old challenges pile up
March 2022—The omicron surge was waning on Feb. 1 when Compass Group members met by Zoom with CAP TODAY publisher Bob McGonnagle. Inpatient numbers, test demand, and positivity rates were declining. “We’re on the downslope,” Northwell Health’s Dwayne Breining, MD, reported. But other pressures persist: the shortages of blood and staff.
Read More »At Penn State, a fast track to pathology residency
March 2022—A new program called Pathology Accelerated Pathway at Penn State shepherds pathology-bound students toward residency readiness in three years instead of four and is set to begin this spring.
Read More »Next-level testing for allergy, autoimmune disease
March 2022—Recent years have seen new releases in allergy and autoimmune disease testing that move the fields forward. “On average we’re looking at six to 10 years of a patient going through the process of seeing specialist after specialist and finally reaching a diagnosis,” Veena Joy, MSc, PhD.
Read More »Molecular oncology tumor board: The curious cases of medullary thyroid cancer
March 2022—Most thyroid cancer cases are straightforward, but a small percentage are not. In a CAP21 molecular oncology tumor board session, Justin Bishop, MD, and Saad Khan, MD, spotlighted one of the latter.
Read More »New illustrated guide to bone marrow based on PT
March 2022—CAP Publications will release this spring volume two of the second edition of the Color Atlas of Hematology—on bone marrow. It continues in the tradition of its predecessor on peripheral blood cells (volume one, 2018): morphologic identification of cells based on proficiency testing. The senior and associate editors have organized the various components of volume two into “an eminently readable, practical, and in many respects entertaining resource for anyone interested in bone marrow morphology, physiology, and pathophysiology,” Donald S. Karcher, MD, of George Washington University, writes in the foreword.
Read More »Connectivity and control—pivotal issues at the POC
March 2022—How can we connect these point-of-care devices? How do we standardize and ensure competency? How do we get the results from at-home testing? How can we integrate point-of-care information into the whole of analytics? Just some of the questions those who lead POC testing and make it possible are asking today. They spoke with CAP TODAY publisher Bob McGonnagle on Jan. 25 in a virtual roundtable on POC instruments and system connectivity.
Read More »Letters
March 2022—This is in regard to the article in CAP TODAY, January 2022, “What influences med students to choose pathology?” which discusses reasons for the low enrollment in pathology residencies. The article is informative but there is no mention of an important resource we established at the CAP Foundation.
Read More »After the switch: high-sensitivity troponin
February 2022—Like growing old gracefully, moving to high-sensitivity cardiac troponin is both easier and more complex than it often appears. Stacy Beal, MD, thought clinical colleagues might be intimidated by switching assays. Dr. Beal was fully prepared to field worries about increased admissions, more consults, and other disaster scenarios. Instead, what surprised Dr. Beal, a member of the CAP Quality Practices Committee, was the ease with which some thought change could occur. “We heard people saying, ‘Just move the decimal point over two spots,’” she recalls. “I think we started hearing that from the day we started talking about it.” Could simply moving the decimal work? As Dr. Beal notes, “It’s hard to argue with that method, but we truly tried to tell them not to—that they needed to interpret this in a totally different way, and to view the different units as a new assay that’s very different from our previous assay.” “Maybe it’s our own fault,” Dr. Beal concedes. When the lab presented its correlation data, the new units were presented on one axis, while the old ones appeared on another.
Read More »Steps to preventing coag test processing error
February 2022—It was Isaac Asimov who surmised: “The most exciting phrase to hear in science is not ‘Eureka!’ but ‘That’s funny . . .’” And it was coagulation processing in the clinical laboratory that, in a small way, illustrated Asimov’s axiom for Dorothy Adcock, MD, former chief medical officer, Labcorp Diagnostics.
Read More »A look ahead at AI-based assistance in anatomic pathology
February 2022—In a survey of the international pathology community on the integration of artificial intelligence into diagnostic pathology practice, 80 percent of the 487 respondents predicted integration within the next five or 10 years.
Read More »Gastric intestinal metaplasia—the need to classify and how
February 2022—How to classify gastric intestinal metaplasia, when to classify it, and the implications of a GIM diagnosis were the focus of a CAP21 presentation in a session on advances in gastric neoplasms. The big question, said presenter Namrata Setia, MD, is, “Why are we suddenly talking about classifying intestinal metaplasia in the stomach?
Read More »Staff out, instruments down—coping as the year begins
February 2022—New year, new variant. For Compass Group lab leaders on a call with CAP TODAY publisher Bob McGonnagle on Jan. 4, omicron was new but the struggles were similar. They spoke of staffing and supplies but also of the CDC’s day-five guidance, crisis planning, instrument downtimes and hard-to-get parts, and doing such things as limiting routine phlebotomy draw times for COVID-positive inpatients.
Read More »Molecular oncology tumor board: Pathologist, oncologist dip into head and neck case
February 2022—Up first in a CAP21 molecular oncology tumor board session was an unusual case of head and neck cancer, one that raises questions about what salivary duct carcinoma is and the role of next-generation sequencing. Justin Bishop, MD, the Jane B. and Edwin P. Jenevein, MD, distinguished chair in pathology and professor and chief of anatomic pathology, UT Southwestern, along with Saad Khan, MD, assistant professor of medicine (oncology) at Stanford, teamed up to present the case, along with a second case that will be reported in the March issue. (Dr. Khan practiced at one time at UT Southwestern, so this wasn’t their first tumor board together.)
Read More »AMP case report: A case of a rare myeloid neoplasm presenting with features mimicking primary myelofibrosis
February 2022—Myeloproliferative neoplasms (MPNs) are a group of clonal hematopoietic stem cell disorders characterized by increased proliferation of myeloid cells of one or more lineage. The most common MPNs include chronic myeloid leukemia (CML), polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). CML is defined by the BCR-ABL1 fusion, which typically results from the t(9;22)(q34;q11.2) rearrangement.
Read More »The rush to deliver integrated reporting in pathology
February 2022—Oracle’s purchase of Cerner, cloud computing, and integrated reporting were up for discussion when CAP TODAY publisher Bob McGonnagle convened a virtual roundtable Jan. 6 on anatomic pathology computer systems. Hematopathologist Monica E. de Baca, MD, said on the call she was encouraged by what she heard about integrated reporting from the AP LIS vendor reps on the call. But she said: “We should also be thinking about what is next; we don’t want to be talking about things 10 years after they were needed.” She and seven others answered McGonnagle’s questions, among them: Are the resources in pathology adequate to make progress toward and enable the necessary IT outcomes?
Read More »Letters
February 2022—The CDC published on Nov. 5, 2021 a report indicating that immunocompromised persons receiving SARS-CoV-2 mRNA vaccines should receive three doses and a booster (MMWR Morb Mortal Wkly Rep. 2021;70[44]:1553–1559). The following n-of-1 observational study suggests that an immunocompromised nonresponder to mRNA vaccine may indeed respond to a second series of SARS-CoV-2 mRNA vaccine.
Read More »A gap in need of a fix: EHRs and genomics
January 2022—“Workflow” evokes a process that moves smoothly, like water, that doesn’t break down or grind to a halt, a sequence of steps that can be completed in a seamless manner. Genomic workflows in information systems, however, have an especially poor fit with the concept of “flow.” As genomic data migrate from the laboratory to an electronic health record or from one EHR to another, significant gaps can result between generation, interoperability, and utilization that may lead the data to miscommunicate or mislead. “The technology behind next-generation sequencing and genetic testing in general has advanced by leaps and bounds over the last 10 to 15 years,” says Alexis Carter, MD, physician informaticist, pathology and laboratory medicine, Children’s Healthcare of Atlanta. While the use of genomic testing has expanded rapidly, “information systems and electronic health records have really not been able to keep up.”
Read More »Measuring direct oral anticoagulants—when, how
January 2022—Laboratories don’t have to monitor direct oral anticoagulants, but they might want to measure DOAC drug levels in some situations in some patients, said Karen A. Moser, MD, in a CAP21 session.
Read More »Labs hunt for solutions to staffing, plastics, and blood supply shortages
January 2022—“Still a pressure cooker and hotter now” and “grim.” Two comments that describe where some states were on Dec. 7 when Compass Group members met for their monthly call on COVID-19 and more—and this was before omicron spread in the United States.
Read More »Why yearly TB testing of health care workers is a waste
January 2022—The United States and the rest of the world can expect to see an uptick in active tuberculosis cases brought about by impaired access to care and delays in diagnosis and treatment during the pandemic, says Wendy Thanassi, MD, MA.
Read More »What influences med students to choose pathology?
January 2022—It’s not curriculum. It’s visibility. That’s the upshot of two companion studies on what influences U.S. medical students to choose pathology as a specialty, say Cindy B. McCloskey, MD, chair of the CAP’s Graduate Medical Education Committee, and Melissa R. George, DO, a member of the committee. Their study of allopathic medical students was published in 2020, and their latest study, of osteopathic medical students, has been submitted for publication.
Read More »AMP case report: Rhinoscleroma in Southern California—diagnosis made by multidisciplinary investigation
January 2022—A 33-year-old male with progressive hoarseness and shortness of breath was given a purported diagnosis of laryngeal papillomatosis and referred to our institution in November 2020 for a higher level of care. On presentation, the patient reported no recent upper respiratory infection-like systemic symptoms but had cough, nasal congestion, throat discomfort, dysphonia, and worsening dyspnea.
Read More »Cytopathology in focus: Special stains in the cytology laboratory
January 2022—A consistent virtue of the cytopathology laboratory is that it combines two qualities essential to patient care: It provides an accurate and timely diagnosis. The ability to make a prompt diagnosis is particularly important in immunosuppressed or otherwise vulnerable patient populations for whom a timely diagnosis can result in early treatment initiation and potentially better outcomes.
Read More »Cytopathology in focus: p16 immunostaining in cytology specimens—a diagnostic pitfall
January 2022—Cytopathologists are often the first pathologists to diagnose HPV-related head and neck squamous cell carcinomas (HNSCC). These head and neck cancers can present as superficial masses amenable to fine-needle aspiration, where p16 immunostaining is used as a surrogate marker for HPV in situ hybridization in a subset of squamous cell carcinomas.
Read More »Cytopathology in focus: Statistical reporting—benefits beyond the numbers
January 2022—The CAP has a robust Laboratory Accreditation Program with a commitment to continually improving the programs and providing appropriate resources needed for compliance. As a deemed status organization, validation surveys are performed annually through the Centers for Medicare and Medicaid Services and the feedback obtained provides direction for education.
Read More »Cytopathology in focus: Our appeal to program participants—return glass slides
January 2022—A diverse and copious inventory of Pap and nongynecologic glass slides is the backbone of the CAP glass slide educational programs. Each year tens of thousands of cytopathology slides are packaged and mailed to laboratories enrolled in CAP educational glass slide programs throughout the world. Prior to mailing, numerous cytotechnologists and cytopathologists screen these slides and companion web enhancement images to ensure quality and diagnostic accuracy.
Read More »eGFR equation no longer Black and white
December 2021—There are success stories. There are overnight success stories. And then there are things that just seem to happen overnight—minus the success. In the midst of chronic discontent over the use of a race coefficient in equations for estimating glomerular filtration rate, one San Francisco hospital sought to make a change. The hope was to help end disparities in health care, such as lower kidney transplantation rates in Black people with chronic kidney disease. The change at Zuckerberg San Francisco General Hospital was pushed by what Neil Powe, MD, MPH, MBA, calls “a very small group of faculty and trainees lobbying the lab, unbeknownst to others.” The lab reported two values, using the eGFR equation with and without the race coefficient, but assigned “high muscle mass” and “low muscle mass” to the two values as a way to step around the troubling race component. Dr. Powe, the hospital’s chief of medicine, doesn’t mince words when he considers how the change was made. “And it stereotyped ethnic groups even more,” he says.
Read More »MMR, MSI testing guideline nears finish line
December 2021—No single assay can capture all cancer patients with DNA mismatch repair deficiency, and in determining a patient’s eligibility for immune checkpoint inhibitor therapy, assays for MMR deficiency, microsatellite instability, and tumor mutation burden should not be considered interchangeable.
Read More »Tight and terrible: Lab leaders on budgets and staffing
December 2021—The staffing crisis lives on, despite labs having plans of all kinds in place to alleviate the shortage. “It’s the only thing we’re talking about,” Ochsner Health’s Greg Sossaman, MD, said on Nov. 2 when members of the Compass Group met by Zoom. SARS-CoV-2 testing and test supplies and vaccination are “taking a back seat” to staffing, he said.
Read More »DELFI approach as ‘pretest’ in early cancer detection
December 2021—A cost-effective liquid biopsy focused on analyzing genomewide fragmentation profiles in cell-free DNA has been shown in proof-of-concept studies to detect early-stage lung and other cancers.
Read More »Medicare adopts new clinical consult billing codes
December 2021—Pathologists will have a new set of Current Procedural Terminology codes to use for reporting pathology clinical consultation services, beginning Jan. 1, 2022. These codes, which the CAP developed through its advocacy work with the American Medical Association CPT editorial panel, were published in the final 2022 Medicare physician fee schedule on Nov. 2.
Read More »CAP accreditation withdraws ANP.10039 from checklist
December 2021—The CAP has decided to temporarily withdraw the anatomic pathology checklist requirement ANP.10039 (Total Fixation Time) from the 2021 checklist edition, and this requirement will no longer appear in customized checklists provided to laboratories and inspectors using the 2021 edition.
Read More »AMP case report: Identification of encephalomyocarditis virus using metagenomic NGS in a patient with acute febrile illness
December 2021—Clinical metagenomic next-generation sequencing (mNGS), the comprehensive analysis of microbial and host genetic material (DNA and RNA) in patient samples, is increasingly available in clinical laboratories. At present, for various reasons including test complexity, cost, and turnaround time, this technology is generally limited to difficult-to-diagnose conditions where conventional microbiological testing methods may not lead to a definitive diagnosis in selected patients, such as those with meningoencephalitis of unknown etiology.
Read More »Urinalysis: Efficiency, utility, and the ‘movement in the field’
December 2021—Four experts met on an Oct. 12 call to talk with CAP TODAY about urinalysis—the newest platforms, what labs need, labor solutions. CAP TODAY publisher Bob McGonnagle asked the questions. Providing their perspectives were Matthew Rhyner, PhD, MBA, Beckman Coulter; Jason Anderson, MPH, MT(ASCP), Sysmex America; Megan Nakashima, MD, Cleveland Clinic; and Keri Donaldson, MD, MSCE, Solvd Health and Penn State. Here’s what they had to say.
Read More »A sizable shift in CNS tumor classification
November 2021—Much has changed since the last WHO classification of central nervous system tumors was published five years ago. Case in point: When the group of authors met in Utrecht, the Netherlands, in late 2019, everyone anticipated two more WHO meetings in Europe to work further on the 2021 classification. Arie Perry, MD, a coauthor on both classifications, says the group photo was cheery. “Everybody was smiling.” The later trips to Europe were canceled because of the pandemic and the group met instead by Zoom. “Now everybody looked grumpy,” he says of a screen shot. Fortunately, “We got everything done, even if it wasn’t quite as pleasant,” says Dr. Perry, professor of pathology and neurological surgery, Department of Pathology, Division of Neuropathology, University of California, San Francisco. The result is the latest WHO classification, which offers dramatic changes of its own. “I’m really excited about the new WHO,” he says. “At first it takes a little getting used to”—like, say, a face mask—“but I think it’s another major advance, just like we had last time.”
Read More »New hope for lab data interoperability
November 2021—Interoperability, a problem of long standing in health care, has a new push and new prospects. Interoperability has become a front-burner issue because it has become increasingly urgent to bring the standardized communication of health care data up to speed.
Read More »Compass Group members on test pre-approvals, staff search and strategies
November 2021—Flexible scheduling to suit family life and new “lab associate” roles—two solutions in progress or in place in labs wrestling with the staffing shortage. That and precision medicine test pre-approvals and utilization were some of what Compass Group members talked about on Oct. 5 in a virtual roundtable led by CAP TODAY publisher Bob McGonnagle.
Read More »Latest checklist takes quality management to next level
November 2021—In the latest edition of the laboratory general checklist, released in September, the requirements of the CAP Accreditation Programs have been edited to be more aligned with CAP 15189 (ISO 15189) accreditation requirements.
Read More »Lab analysis in diabetes — a preview of what’s to come
November 2021—The guidelines for laboratory analysis in the diagnosis and management of diabetes mellitus are being revised and will be released next year. In a virtual session at the AACC meeting in September, laboratorians got a look at some of the recommendations to come.
Read More »For genomic testing, a homegrown software solution
November 2021—At NorthShore University HealthSystem in suburban Chicago, a homegrown bioinformatics solution is the “secret sauce” that powers the health system’s personalized medicine program, says Kamalakar Gulukota, PhD, MBA, director of NorthShore’s Center for Bioinformatics and Computational Biology.
Read More »AMP case report: A patient with an unexpected cancer predisposition syndrome—somatic tumor mutation testing and germline mutation testing complement each other
November 2021—Molecular analysis of advanced stage tumors has become the gold standard for identifying potential targetable mutations with high sensitivity, even in limited size tissue samples. However, when only tumor tissue is sequenced, it is difficult to differentiate between somatic mutations in the tumor cells versus constitutional (germline) mutations.
Read More »Security in the cloud leads off in LIS exchange
November 2021—Cybersecurity and the cloud, COVID care gaps, and lab consolidation were among the topics CAP TODAY publisher Bob McGonnagle talked to LIS vendors and Toby Cornish, MD, PhD, about in a Sept. 20 virtual roundtable. A return to on-site trade shows, too, came up: “I do miss walking the vendor floor. I feel like I’m out of touch with what the developments are,” said Dr. Cornish of the University of Colorado Anschutz Medical Campus.
Read More »Results release: new steps under new rules?
October 2021—Neither pathologists nor laboratories should panic over the new 21st Century Cures Act rules making laboratory results immediately accessible to patients, pathology leaders agree. Most laboratories already release results to electronic health records and those results are made available in patient portals, and the Cures Act will require little change in how labs send results to EHR systems. But the rules, which took effect April 5, do come with some complexities to navigate. By passing the Cures Act in 2016, Congress aimed broadly to increase interoperability across EHR platforms and to ensure that patients have full, portable, and cost-free access to their health care information. Of most direct relevance to pathology is the Cures Act’s information blocking or open notes rule, mandating that lab report narratives and pathology report narratives, along with six other categories of clinical notes, be available without delay to patients in different electronic formats, including smartphones and secure online portals.
Read More »Parsing the role of race in Alzheimer’s biomarkers
October 2021—It’s not quite six degrees of Kevin Bacon, but the connection between Alzheimer’s disease biomarkers and equity in medicine is real (and far more important). It’s a trail researchers have been following for some time, but which has gained more prominence with the recent approval of a new drug for treating the disease (aducanumab) and the acknowledgment of racial disparities in CSF amyloid and tau biomarkers and their associated cutoffs.
Read More »AST and safety at core of microbiology checklist changes
October 2021—By Jan. 1, 2024, laboratories must use current breakpoints to interpret antimicrobial minimum inhibitory concentration and disk diffusion test results, according to a new requirement in the latest edition of the CAP Accreditation Programs microbiology checklist, released Sept. 22.
Read More »Up close on clonal hematopoiesis in cfDNA testing
October 2021—Clonal hematopoiesis is a significant biological phenomenon and denotes presence of mutations in bone marrow stem cells in the absence of a hematologic malignancy.
Read More »Molecular or morphology? Challenges in pathologic diagnoses
October 2021—Recent molecular genetic advances have dramatically expanded diagnostic options, thus revolutionizing the diagnosis of many tumor types, especially those of soft tissue and bone. Advances in the discovery of molecular alterations underlying neoplastic pathogenesis have also provided insights into novel therapeutic targets and prognostic biomarkers. These improvements have led to the reclassification of a growing list of previously established tumor types, resulting in significant challenges for practicing pathologists, as exemplified herein.
Read More »‘Scary situation’—lab leaders on staffing and COVID
October 2021—Surge, supplies, staffing. Eighteen months into the pandemic, the story remains similar. Even where laboratory salaries have been bumped up or sign-on bonuses have been in place to strengthen the workforce, Compass Group members report little to no success. And on supplies: “Every week we cross our fingers and toes to see what arrives in the door, how to disperse that through the systems, and how to continually educate the physicians on appropriate use of that limited resource,” says Judy Lyzak, MD, MBA, of Alverno Laboratories. She and other laboratory leaders of the Compass Group met virtually Sept. 6 to share their latest. Of the confluence of problems laboratories face, one said: “I have never seen anything quite like this.”
Read More »Detecting myeloid malignancy minimal residual disease
October 2021—Detecting leukemic cells for post-treatment monitoring in normal karyotype acute myeloid leukemia is challenging, but new approaches to minimal residual disease monitoring may make it increasingly possible in the clinical laboratory, David Wu, MD, PhD, said in an AMP webinar he presented recently on myeloid malignancy minimal residual disease detection.
Read More »Panel weighs in on practices, pressures in heme labs
October 2021—Rules, slide reviews, test ordering, and provider education were part of the conversation when CAP TODAY publisher Bob McGonnagle convened a hematology-focused virtual roundtable in late August. Workforce problems too: “We have a bigger exodus now and our pipeline is smaller,” said Eric D. Hsi, MD, of Wake Forest University.
Read More »In coag collections, every detail counts
September 2021—Rare wine? Delectable. Rara avis? Magnificent. Rare blue-top collection tube? Uh oh. For Richard Marlar, PhD, coming across a non-FDA-approved tube was an unhappy discovery. Dr. Marlar, medical director, coagulation laboratory, University of New Mexico Hospital, says his lab was among the first to encounter one of these rogue tubes, available for purchase on the internet and likely taking wing due to pandemic supply shortages. When the tube arrived for testing, it quickly kindled concerns, says Dr. Marlar. “It’s a tube we had never seen before. It looks like it has a CE mark on it, and the Europeans don’t know anything about it. It has a label on it that suggests it’s FDA approved—but the FDA is not aware of it,” he says, adding that his lab has spoken with the agency. It feels like a “CSI”-tinged moment in a venue that labs would prefer to keep drama-free. It also points to the ongoing need to keep a keen eye on what passes through coagulation laboratories. It’s not so much that the devil is in the details; rather, that’s where accurate results lie.
Read More »Gastric HER2, hsALK to join monitored PT list
September 2021—Beginning next year, CAP-accredited laboratories that perform HER2 immunohistochemistry in gastroesophageal adenocarcinoma or highly sensitive (hs) ALK in non-small cell lung cancer will be required to enroll in proficiency testing for those analytes.
Read More »Metagenomic NGS: More pros than cons?
September 2021—A stem cell transplant patient at Lurie Children’s Hospital in Chicago had a disseminated fungal infection by every clinical criterion, but no conventional method had detected it.
Read More »How to prevail over a crisis using data analytics
September 2021—The pandemic has pelted hospitals and laboratories with wild cards and sometimes thrown wrenches into the works. But the jolts it has delivered to normal institutional operations, forcing new solutions to business and clinical care dilemmas, have also positioned laboratories to help produce stunning new capabilities.
Read More »A preanalytics push in accreditation checklists
September 2021—Taking steps to protect the integrity of specimens is at the heart of new and revised requirements in this year’s edition of the accreditation program checklists, set for release Sept. 22. A CAP team made up of members of the Checklists, Personalized Health Care, and Cytopathology committees collaborated to incorporate into the checklists the evidence-based recommendations set forth in a 2019 article on preanalytics and precision pathology. Many of the new and revised requirements, which are in seven checklists, are aimed at improving the quality of tissue and blood specimens that may undergo molecular testing for patients with cancer. The aim of others is to improve the preanalytic quality of specimens used for all types of testing.
Read More »Salaries, schools, students—all eyes on workforce
September 2021—SARS-CoV-2 spread and the staffing shortage drove the conversation when Compass Group members met Aug. 3 for their monthly call led by CAP TODAY publisher Bob McGonnagle. “Like others, we were seeing problems before COVID, but COVID seems to have kicked it into overdrive,” Steven Carroll, MD, PhD, of the Medical University of South Carolina, said of the shortage. And more long term, it’s time to jump-start training programs, he and others say. The Compass Group is an organization of not-for-profit IDN system laboratory leaders who collaborate to identify and share best practices and strategies. Here is what they shared last month.
Read More »For MammaPrint and BluePrint, the long-term view
September 2021—The latest data on the use of two genomic assays in early-stage breast cancer and at the University of Rochester Medical Center as the pandemic set in were reported in a CAP TODAY webinar presented by William Audeh, MD, and David G. Hicks, MD. Dr. Audeh, medical oncologist and chief medical officer of Agendia, developer of MammaPrint and BluePrint, presented the long-term follow-up results of the MINDACT trial and an age-related analysis, as well as new data on MammaPrint’s use in endocrine therapy decisions.
Read More »AP lab maps its cyberattack recovery
August 2021—The downtime manual that the anatomic pathology laboratory at the University of Vermont Medical Center maintained in 2020 was never intended to be used for dealing with a cyberattack. In fact, it wasn’t actually a manual. It was a laboratory-wide policy essentially consisting of one instruction to be used in the event of a power failure or short-term IT disruption or other emergency: “Bring everything to a halt.” In anatomic pathology, “Our downtime protocol was: You stop in your tracks,” says dermatopathologist Anne M. Stowman, MD. “For the urgent/emergent specimens, you get out your paper logs, you do paper recording of the cases coming in, and you handwrite your cassettes, your descriptions, your slides.” That would be a bit slower and less efficient, but it would work for brief, temporary outages and disruptions. But the cyberattack that UVMMC experienced in October 2020, cutting off the labs’ access to the medical center’s information technology systems and disabling operations for more than three weeks, was an abrupt wake-up call.
Read More »CSF biomarkers in ‘a new era’ for Alzheimer’s
August 2021—The FDA’s approval in June of a new drug to treat Alzheimer’s disease has incited motley responses, a sort of Grand Tour of emotions and reactions. As with any stormy action, the aftermath becomes clear only over time, during the hard work of cleaning up. What comes next?
Read More »Lab staff shortage calls for speed, money, and more
August 2021—From critical to worse is how two Compass Group members described the lab labor shortage on July 6 when the group spoke with CAP TODAY publisher Bob McGonnagle in another of their monthly virtual meetings. “Things imploded. We’re struggling in every area,” said one.
Read More »Testing for platelet function using whole blood
August 2021—Platelet function testing using platelet-rich plasma is the gold standard, but whole blood platelet aggregation has its advantages, with less risk for preanalytic error among them.
Read More »National patient identifier advocates state their case
August 2021—The iconic Timex slogan “It takes a licking and keeps on ticking” was designed to sell watches in the 1950s and ’60s, but it could just as easily be applied to efforts to repeal the decades-long appropriations ban on a unique national patient identifier—year, after year, after year.
Read More »PGx in transplant medicine: tacrolimus as foundation
August 2021—Pharmacogenetic testing is not standard of care at most transplant centers, nor do the FDA or others mandate it. But “transplant medicine is ripe for observing the benefits of pharmacogenetics,” said Gwendolyn A. McMillin, PhD, D(ABCC), in an AACC virtual session last year.
Read More »AMP case report: Celiac genetic health risk screening by NGS in the family of a child with clinical findings of dermatitis herpetiformis and gluten sensitivity
August 2021—Dermatitis herpetiformis (DH) is an autoimmune skin disorder associated with celiac disease and characterized by pruritic, blistering lesions mainly on the elbows, knees, buttocks, lower back, and scalp. Immunopathogenesis is attributed to IgA TG3 antibody immune complexes in the papillary dermis originating from subclinical celiac disease in the gut. Onset occurs at any age, but DH is diagnosed most often in young adults and rarely in children. Individuals of northern European heritage represent the most frequently affected population for DH, with an estimated incidence reported by the National Organization for Rare Disorders of 75.3 per 100,000 people. First-degree family members of patients with DH are at increased risk for both DH and celiac disease.
Read More »Cytopathology in focus: Reflections on use of Milan System, edition 1: Areas to be explored for edition 2
August 2021—Salivary gland neoplasms (SGN) are a special group of tumors due to the high variation in histologic subtypes that are further complicated by frequent overlapping morphological features. Fine-needle aspiration is a safe, cost-effective, first-line modality for diagnosing SGNs, an integral part of SGN preoperational workup. In 2018, Faquin and Rossi led the effort to standardize the reporting system of salivary gland lesions. Their final product, Milan System for Reporting Salivary Gland Cytopathology (MSRSGC), has had a huge impact on salivary gland FNA practice in the United States and worldwide.
Read More »Cytopathology in focus: At the center of AI implementation in cytopathology
August 2021—Recent advances in the deep learning area of artificial intelligence offer tantalizing opportunities to improve cytology practice. However, aside from the commercially available options for automated screening in gynecologic cytology, systems with applications in cytology have largely been used in research settings only. The article by authors McAlpine and Michelow reviews the approach to developing and validating artificial intelligence algorithms in cytology, from the generation of appropriate cytology data sets to clinical validation of the model.
Read More »Coag issues occupy COVID’s central stage
July 2021—The pandemic’s reach has often been portrayed in shades of red, signaling surging COVID-19 cases across states and countries. Vaccination maps, on the other hand, tend to render progress in more soothing tones, typically in the green family. But in coagulation laboratories, one small portent is colored blue—specifically, blue-top sodium citrate tubes. In recent months, laboratories began voicing concerns about tightening supplies. They’ve spoken with their vendors; some have reached out to new ones. And though no one wants to think about limiting testing if supplies truly become scarce, it wouldn’t be the first time labs have had to steer through these waters. The tubes are a functional symbol of the continued complexities of COVID-19-related coagulopathy, as physicians try to understand and respond to the pathophysiology of infection that leads to a thrombotic event. As the pandemic has churned on, much has started coming into sharper focus. Prepublication persists, but physicians have begun to sort through the past 18 months and, as many have put it, to “do the science.”
Read More »Analytics reframes decisions from bench to C-suite
July 2021—From takeout margaritas to the embrace of remote work, the pandemic upended convention, leaving behind permanent changes that were nowhere on the radar in 2019. In the world of pathology informatics, the new online COVID-19 data dashboards at the Cleveland Clinic illustrate how much the pandemic has raised the profile of data analytics in managing the laboratory.
Read More »Accuracy-Based Programs for ‘bigger dividends,’ better care
July 2021—Proficiency testing is the bedrock of good laboratory performance, but Accuracy-Based Programs are equally important, which is why members of a CAP committee are hoping for 10 labs from each peer group to participate.
Read More »A few years in, a new picture for liquid biopsy
July 2021—Liquid biopsy has entered a more confident era, with two FDA-approved next-generation sequencing assays for comprehensive tumor mutation profiling, evidence of its clinical utility, and broadened patient access.
Read More »Variants, vaccine efficacy, and the tests labs need
July 2021—For SARS-CoV-2, there has to be a plan to sequence locally and collaborate globally, and public health must recognize that hospital-based laboratories have a part to play, says Glen Hansen, PhD, D(ABMM), medical director of microbiology and molecular diagnostics at Hennepin County Medical Center, Minneapolis.
Read More »Lab leaders on growth, labor, and cybersecurity
July 2021—Revenue and growth, cybersecurity, and labor and wage pressures were on minds June 1 when Compass Group members met virtually with CAP TODAY publisher Bob McGonnagle. But perhaps no problem felt heavier than the labor shortage.
Read More »Testing for platelet function using platelet-rich plasma
July 2021—Identifying severe disorders of primary hemostasis is relatively straightforward for most coagulation laboratories, but the more prevalent disorders with less severe bleeding and less overt diagnostic abnormalities are trickier, and platelet function testing using platelet-rich plasma remains the gold standard. Geoffrey Wool, MD, PhD, in an AACC virtual session last year, presented some of his laboratory’s cases to illustrate the use of light transmission aggregometry and a modification called lumi-aggregation.
Read More »AMP case report: MYC amplification identified in an EML4-ALK-positive lung adenocarcinoma with primary resistance to targeted therapy
July 2021—The advent of genomically targeted therapy and immunotherapy has greatly altered the clinical management of advanced non-small cell lung cancer (NSCLC). Molecular testing is recommended for sensitizing EGFR mutations, ALK fusions, ROS1 fusions, BRAF V600E, NTRK fusions, RET fusions, and MET exon 14 skipping alterations.
Read More »Close ties: instruments, middleware, and more
July 2021—Laboratory instrumentation from an IT perspective and as one solution to the labor shortage were the topics explored April 27 in a virtual roundtable of instrument vendors and laboratory medical directors, led by CAP TODAY publisher Bob McGonnagle. Part one of their conversation about core labs was published in the June 2021 issue; part two follows.
Read More »Under one cover: grossing, staging, and reporting
July 2021—CAP Publications released this month Grossing, Staging, and Reporting—An Integrated Manual of Modern Surgical Pathology, edited by Qihui “Jim” Zhai, MD, professor of laboratory medicine and pathology, consultant pathologist, and director of the FISH laboratory, Mayo Clinic Florida. The manual consists of 12 sections—from breast to thorax—and 54 chapters, organized by organ. CAP TODAY asked Dr. Zhai to tell us about the new book.
Read More »Letters
July 2021—The article about eGFR (“A transparent lens on estimated GFR,” June 2021) reinforces my initial attitude about eGFR: that it is an inaccurate contrived calculation. Testing for the status of a patient’s renal function is information too crucial to be done as a shortcut even though the National Kidney Foundation claims that eGFR is the most accurate way of determining renal function.
Read More »Higher stakes in systemic mastocytosis
June 2021—Mastocytosis is not for quitters. Not at any point, from considering the possible diagnosis, to doing a complement of stains, to looking for mutations beyond KIT D816V, to being curious about the presence of mast cells even after making a diagnosis of another myeloid disease. Patients have already learned this grueling lesson. They can easily spend years seeking answers before their disease is properly identified. Pathologists can speed up that process—and the time to do so is now, says Tracy George, MD, chief medical officer and incoming president of ARUP Laboratories, and medical director of hematopathology. Notes Dr. George: “There’s some exciting stuff going on with systemic mastocytosis.” New targeted KIT inhibitors appear to be quite effective, including at least one agent for advanced systemic mastocytosis that has been submitted to the Food and Drug Administration. “We anticipate there’s going to be approval by the FDA this summer,” says Dr. George, who’s been involved in the clinical trials for avapritinib (Blueprint Medicines).
Read More »1- or 2-step: Outcomes studied in GDM screening
June 2021—If screening for gestational diabetes mellitus were a dance competition, it might have a contest between quickstep and paso doble as its signature event. That tournament could pit the one-step testing protocol (twice as likely to diagnose GDM) against the two-step testing protocol (significantly easier for pregnant women to adhere to).
Read More »Puzzling out platelet function disorders
June 2021—In an AACC virtual session last December, Catherine P. M. Hayward, MD, PhD, of McMaster University, set out the stepwise approach to testing for platelet function disorders, explained the methods used to assess platelet aggregation response, and reported what most clinical labs do.
Read More »Virtual, blended inspections a sign of the times
June 2021—As COVID-19 restrictions halted traditional laboratory inspections, virtual and blended inspections became the stand-ins, and early adopters say there’s much to like and hold on to post-pandemic.
Read More »pTX and pNX should not be used in tumor staging
June 2021—For the June 2021 release of updates for the CAP cancer protocols and the corresponding electronic cancer checklists used by electronic health record vendors, pTX and pNX will no longer be selectable options for use by pathologists when assigning pathologic staging based on definitive surgical resection (pTNM). This change is coming after extensive discussions with the American Joint Committee on Cancer (AJCC) and with its unanimous endorsement. Why are we making this change?
Read More »Compass group roundtable: ‘Gaps loom large’: labor shortage hitting hard
June 2021—A brief update on SARS-CoV-2 variant testing and then a look at the latest on the laboratory labor struggle. That’s what Compass Group members provided when they spoke May 4 in another of their monthly calls led by CAP TODAY publisher Bob McGonnagle. “We’ve accepted that if we’re going to solve the [labor] issue,” said Sam Terese of Alverno Laboratories, “we’ll have to create the workforce. They’re not coming to us in any other way.” With McGonnagle and Terese were Bob Stallone and James Crawford, MD, PhD, Northwell; Sterling Bennett, MD, MS, Intermountain; John Waugh, MS, MT(ASCP), Henry Ford; Peter Dysert, MD, Baylor Scott & White; Steven Carroll, MD, PhD, Medical University of South Carolina; Stan Schofield, MaineHealth; Gregory Sossaman, MD, Ochsner; Clark Day, Indiana University; Diana Kremitske, MS, MHA, MT(ASCP), Geisinger; Julie Hess, AdventHealth; Terrence Dolan, MD, Regional Medical Laboratory; and Dan Ingemansen, Sanford. The Compass Group is an organization of not-for-profit IDN system lab leaders who collaborate to identify and share best practices and strategies. Here is what they said.
Read More »ED, lab views on point-of-care cardiac troponin
June 2021—Point-of-care cardiac troponin testing got a fresh look last December when an emergency medicine physician and a clinical chemist came together to talk about the use of both conventional POC troponin assays in a high-sensitivity era and high-sensitivity POC troponin testing when it becomes available.
Read More »B- and T-cell neoplasm features and fine points
June 2021—A case of monoclonal B-cell lymphocytosis and a tour of B- and T-cell morphologies were at the heart of a CAP20 virtual presentation on neoplastic lymphocytosis. Kyle Bradley, MD, associate professor of hematopathology and director of surgical pathology at Emory University, spoke last fall on reactive (CAP TODAY, May 2021) and neoplastic lymphocytosis, with Olga Pozdnyakova, MD, PhD, who addressed neutrophilia and monocytosis (CAP TODAY, February and March 2021). Together they took attendees through a morphology-based approach to hematopoietic neoplasms presenting with an abnormal WBC differential.
Read More »Transplant viral monitoring struggles and solutions
June 2021—Testing for viral infections post-transplant is an important part of care for transplant patients because of the risk for infections during immunosuppression. But viral load monitoring suffers from interlaboratory variability for several reasons, and while the problem is greater at higher viral loads, the interlaboratory variability is also present at lower viral loads. Steve Miller, MD, PhD, of the University of California San Francisco, and Joseph Yao, MD, of Mayo Clinic in Rochester, reported the monitoring difficulties, solutions, and new directions last fall in a CAP TODAY webinar made possible by a special educational grant from Roche.
Read More »Next moves for core labs—panel takes stock
June 2021—Pause and restart, or rethink and reorient? That’s the question CAP TODAY publisher Bob McGonnagle put to instrument vendors and James Faix, MD, and David Grenache, PhD, D(ABCC), about COVID and core labs and the instruments in those labs. What impact the pandemic had on them and their customers was a topic of discussion when they met on an April 27 call during which they talked, too, about antibody testing and the proliferation of SARS-CoV-2 testing labs during the pandemic. What follows is part of their conversation. The rest, on IT and the staffing shortage, will be published in July, as will our guide to chemistry and immunoassay analyzers for mid- to high-volume labs.
Read More »A transparent lens on estimated GFR
May 2021—Forget about who’s buried in Grant’s Tomb (though for the record it’s Grant, his wife, and their dog). For laboratories, the deceptively simple question now under scrutiny is, What is estimated GFR? It is indeed an estimate, for starters—an approximation of glomerular filtration rate, which in turn is a physiological parameter that’s actually difficult to measure, says Greg Miller, PhD. Even so-called measured GFR values are not very precise in individual patients. It’s been carried along by several equations over the decades: Cockcroft-Gault, MDRD, and CKD-EPI, all of which (to the consternation of some) are still in use. It guides clinical care, including referrals to specialists and placement on kidney transplant lists, as well as dosing of medications such as metformin. Some call it a workhorse. But estimated GFR (eGFR) has also long been saddled with a race-based component, a coefficient that adjusts for better kidney function for Black patients compared with other patients.
Read More »On the frontline of health care cybersecurity
May 2021—In the best of times, the health care industry has been the one most targeted by cyberattacks in the past decade. The pandemic has made health care an even more inviting mark, increasing the urgency of adopting effective cybersecurity measures.
Read More »Lymphocytosis: distinguishing benign from malignant
May 2021—How to distinguish “reactive” and “nonreactive” benign lymphocytosis from malignant lymphocytosis, and between benign and malignant large granular lymphocytosis, is how Kyle Bradley, MD, of Emory University, opened his talk in a CAP20 virtual session last fall.
Read More »Resistance targets: blood culture ID panel pitfalls
May 2021—Most of the time, bloodstream infection antimicrobial resistance results achieved with blood culture molecular ID panels will be accurate. When and why they might not be was the focus of an AMP 2020 virtual session. “I don’t want to lead anyone to believe that these are not good, accurate, and important types of tests,” Richard E. Davis, PhD, D(ABMM), MLS(ASCP)CM, said of the panels.
Read More »Lab leaders on variant testing and result requests
May 2021—How variant testing is being handled and how labs should respond to clinicians’ requests for the results was a topic of discussion when Compass Group members met April 6 with CAP TODAY publisher Bob McGonnagle for their monthly roundtable on COVID-19. The group’s members provided a follow-up on post-vaccination infections and reports on pre-procedure testing, and their thoughts on whether the focus has shifted away from testing amid the press to vaccinate. Until it’s known whether the U.S. can keep pace with vaccination alone, “it’s a mistake to take our eye off of what testing can offer, especially in terms of variant detection,” said Sterling Bennett, MD, MS, of Intermountain Healthcare.
Read More »NGS in more labs? IFCC group aims to ease the way
May 2021—When it comes to next-generation sequencing, don’t count out community hospital labs, especially as black-box solutions come on the market. That’s the hope of members of an International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) working group that aims to help clinical labs develop in-house NGS programs. Large-scale genomic testing won’t be necessary or practical at the community hospital level. But hospital-based genomic testing programs should set out to meet the NCCN guideline targets and provide testing for which a wide range of sample input and quality can be accepted, says Robyn Sussman, PhD, a member of the IFCC working group and molecular development assistant director, Penn Precision and Computational Diagnostics, University of Pennsylvania Perelman School of Medicine.
Read More »Eyes on faster, cheaper, simpler next-gen sequencing
May 2021—Next-generation sequencing analysis and interpretation, as well as reimbursement, were some of what CAP TODAY publisher Bob McGonnagle asked Illumina and Thermo Fisher executives and Jeremy Segal, MD, PhD, about when they gathered on a March 24 call. McGonnagle asked, too, about variants of unknown significance and for views on what lies ahead for NGS. “Circulating tumor DNA analysis is starting to move wholesale into the academic setting,” along with other applications, says Dr. Segal, of the University of Chicago.
Read More »Cytopathology in focus: Inspection pitfalls: Common cytology lab-related deficiencies
May 2021—As COVID-19 restrictions ease, many laboratories are ramping up for biennial CAP inspections. Some of these inspections were delayed due to COVID restrictions and others were performed virtually and now must complete the statutory requirement of an on-site inspection. To add to the mix, the CAP published its 2020 checklist edition earlier than usual because of its impending reapplication with the CMS for deeming authority as an accrediting organization under CLIA. Together, these have made the 2021 inspection process appear unusually daunting. While no laboratory is immune to inspection anxiety, it does help to arm oneself with the knowledge gathered from the collective experiences of peers and colleagues across the country. Knowing what the common inspection pitfalls are can bring us a step closer to the “utopia” of a flawless inspection.
Read More »Cytopathology in Focus: What breast cytology brings to rapid assessment clinics
May 2021—During the past several years, significant changes have occurred in the approach to the diagnosis and follow-up of patients with breast cancer. The scattered and fragmented breast health services have been replaced by patient-centered clinical breast units and rapid assessment breast clinics all over the world.Pioneered and implemented in European countries, rapid assessment breast clinics are designed to effectively assess symptomatic women with palpable breast lesions by fine-needle aspiration biopsy (FNAB).
Read More »Weeks of lab turmoil follow cyberattack
April 2021—After he finished interviewing for a fellowship one morning last October at the University of Vermont Medical Center, pathology resident William O. Humphrey, MD, checked in to attend grand rounds virtually. Then the cyberattack struck. It began mysteriously, with people dropping one by one off the Zoom screen and emails arriving only intermittently. Internet service grew patchy and a hospital staffer unmuted and canceled grand rounds, saying, “We aren’t really sure what’s going on.” From there, a cascade of failures indicated serious trouble. “All of a sudden we’re realizing we can’t sign into our EMR. We can’t get into our email either. My phone isn’t working on the Wi-Fi. Something is wrong,” recalls Dr. Humphrey, a member of the CAP Informatics Committee. That was the prelude to a siege in which fax machines and penmanship were unretired from obsolescence, paperlessness became a relic of the past, and words like “runners” and “bouncers” entered routine laboratory vocabulary.
Read More »Seeking stability in gene nomenclature
April 2021—Human first names are not necessarily known for being meaningful—or unique for that matter. When Shakespeare’s Juliet muses, “What’s in a name?” she’s observing that her lover’s name is more or less an arbitrary label without relevance to the essence of Romeo.
Read More »‘Know your panel’: Blood culture ID cautions
April 2021—The interpretive challenges of blood culture identification panels were the focus of an AMP2020 virtual presentation on false-positives and false-negatives and their sources and solutions.
Read More »SARS-CoV-2 testing: Buy, build, and borrow? Middleware solutions
April 2021—Whirlwind timelines, novel problems, and a never-ending workload: On the anniversary of the COVID-19 pandemic, pathology informatics leaders David S. McClintock, MD, and Christopher Williams, MD, reflected on the year’s onslaught of challenges.
Read More »Calm before spring storm? Compass on COVID
April 2021—Test volumes and positivity rates were down and vaccinations and interest in variants were up on March 2 when Compass Group laboratory leaders met with CAP TODAY publisher Bob McGonnagle for another in a series of calls about SARS-CoV-2. Also in the discussion: antigen and serologic testing, school and sports team testing, and testing for travel.
Read More »From manual to staging system—AJCC’s new path
April 2021—In the continuum of cancer care, diagnostic specialties such as ours play critical roles. We touch virtually all aspects of cancer care through cancer diagnostics, the use of molecular studies in treatment and as predictive markers, and our key role in anatomic pathologic staging. The widespread use of the CAP cancer protocols has improved cancer reporting by standardizing format and terminology while also incorporating the concept of human factors engineering and standards for developing high-quality clinical practice guidelines.
Read More »Billing, business, win, lose: roundtable dives in
April 2021—A look at laboratories post-pandemic was at the heart of a revenue- and billing-focused roundtable led by CAP TODAY publisher Bob McGonnagle on Feb. 10. With McGonnagle were Mick Raich, Vachette Pathology; Bob Dowd, NovoPath; Kwami Edwards, Telcor; Kyle Fetter, Xifin; and Tom Scheanwald and Matt Zaborski, APS Medical Billing.
Read More »Problems, solutions at core of UTI, C. diff modules
April 2021—Urinary tract infections and Clostridioides (Clostridium) difficile testing are the topics of two of the modules released recently in the CAP Test Ordering Program. The Laboratory Workup for Urinary Tract Infections module became available online in January, and C. difficile Testing in October 2020 (www.cap.org/member-resources/test-ordering-program). The program is free to CAP members.
Read More »Looming unknowns with SARS-CoV-2 variants
March 2021—Listening to experts and others make predictions about the pandemic, it’s easy to think they’re obsessed with surfing: How will we deal with the next wave? The dangers are real. With SARS-CoV-2, the next wave might be swept in by emerging variants, with their uncertain but worrisome impact on transmission, severity of illness, treatments, and vaccine effectiveness.
Read More »Close-up on abnormal monocyte morphology in peripheral blood smears
March 2021—A recently published study of 90 patients found significant numeric and atypical white blood cell morphologic changes associated with SARS-CoV-2 infection that differed between mild and severe disease.
Read More »Vascular changes in lung—a piece of the puzzle
March 2021—If you see vascular changes, report them. That is the advice Kristen L. Veraldi, MD, PhD, pulmonary critical care physician, University of Pittsburgh Simmons Center for Interstitial Lung Disease, gave last fall in a CAP20 virtual session on vascular changes in lung biopsies—when and what to report.
Read More »‘The stakes are high’—taking on workplace well-being
March 2021—A focus on workplace well-being is worthwhile all the time but never more so than now. “Everyone knows health care worker fatigue is amplified during the pandemic,” says Sarah M. Bean, MD, professor of pathology and pathology medical co-director of the dermatology/pathology clinical research unit, Duke University School of Medicine.
Read More »One year later, life in labs remains a juggling act
March 2021—Tackling staff shortages, urging vaccination, answering questions about serology testing, and raising questions about variants were some of what Compass Group members were doing when they spoke on Feb. 2 about COVID-19. “Staffing has continued to be a challenge, the greatest of which is phlebotomy staffing,” said Darlene Cloutier, MSM, MT(ASCP), HP, director of laboratory operations at Baystate Health, Springfield, Mass. “Our biggest pain point is laboratory technologists,” said Tony Bull, executive director, AdventHealth, Orlando, Fla. CAP TODAY publisher Bob McGonnagle led the roundtable.
Read More »Tox testing challenges in adolescents, young adults
March 2021—Synthetic cannabinoids. Toxic household substances. Over-the-counter medicine. These and other drug and non-drug substances are favored by adolescents and young adults and tough to detect, and the pandemic has exacerbated their use. “Many drugs that are of interest for this age group are household substances, things they can get their hands on easily, and often those tests are not available as immunoassays,” said Sarah E. Wheeler, PhD, medical director of the automated laboratory, University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh, and assistant professor of pathology, University of Pittsburgh, in a session on adolescent and young adult substance abuse testing at AACC’s virtual annual meeting last year.
Read More »Rapid ID from positive blood culture: Labs tally gains
March 2021—Fresh from its Dec. 27, 2020 FDA clearance, the Bruker MALDI Sepsityper Kit US IVD promises to provide microbiology laboratories with a universal, rapid sepsis identification solution. With the Bruker MALDI Biotyper platform’s reference library covering 491 organisms, the Sepsityper’s ability to identify pathogens directly from positive blood cultures in suspected bacterial or fungal sepsis cases delivers an “order of magnitude increase” in the number of microorganisms that can be identified through PCR detection, said Wolfgang Pusch, Bruker Daltonics executive vice president of microbiology and diagnostics, in a company statement.
Read More »AMP case report: A CLL/SLL case with distinctive molecular and cytogenetic changes during different stages of disease progression
March 2021—Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is one of the most common lymphoproliferative diseases. It is a CD5-positive B-cell neoplasm of monomorphic small mature B cells. One of the characteristics of CLL/SLL is its heterogeneity, not only among individuals but also within individual patients.1 The cytogenetic and molecular variants are dynamic during disease progression and in response to targeted therapies.
Read More »Markers, methods remake the NSCLC map
February 2021—Absorbing new biomarkers into lung cancer workups makes for a complicated diplomacy. How best to balance so many rivals? Does it make the most sense for laboratories to try to do everything at once, a full-court press involving next-generation sequencing panels? Or is it more practical to add a new marker only as a new targeted therapy receives approval? Where do RNA-based assays fit in? What about IHC? When do you make the switch? Or do you? And how best to handle cell-free DNA tests (which seem to be the rogue states in all this)? How do you weight external factors, such as reimbursement, existing equipment and capital expenditures, and physician expertise? Driving this all are medical breakthroughs. As with all forms of statecraft, the latest incident can change everything. For lung cancer, the most recent advance comes from the ADAURA trial, which showed a significant benefit of using osimertinib to treat stage IB to IIIA EGFR-mutation positive non-small-cell lung cancer.
Read More »Setting sights on a coordinated testing strategy
February 2021—From the first, the COVID-19 pandemic has been marked by absence. Reagents and instruments, collection tubes and pipette tips; enough technologists to handle the overwhelming workloads; sleep—so much went missing or was in short supply, month after month.
Read More »In transplantation, detecting CMV antiviral resistance
February 2021—Eighteen months after introducing a next-generation sequencing assay to detect CMV antiviral resistance in the transplant population, Matthew Binnicker, PhD, D(ABMM), in an AMP presentation, shared a patient’s case and his laboratory’s broader experience.
Read More »New data on reference ranges for transgender men
February 2021—Cisgender male reference intervals can be used to interpret testosterone concentrations in transgender and nonbinary adults on masculinizing therapy, but reference intervals specific to the transmasculine population should be used to evaluate estradiol, say the authors of a recently published study.
Read More »Variants, vaccines, predictions: Compass on COVID
February 2021—Variants and vaccines were in the news when Compass Group members spoke with CAP TODAY publisher Bob McGonnagle for the first time in the new year, on Jan. 5. “This is something we all need to stay close to,” Julie Hess, of AdventHealth, said of the variants. “We need to know if it’s going to impact our ability to detect.” With McGonnagle and Hess on the Jan. 5 call were Dwayne Breining, MD, and James Crawford, MD, PhD, Northwell; John Waugh, MS, MT(ASCP), Henry Ford; Stan Schofield, MaineHealth; Gregory Sossaman, MD, Ochsner; Peter Dysert, MD, Baylor Scott & White; Steven Carroll, MD, PhD, Medical University of South Carolina; Heather Dawson, Allina; Janet Durham, MD, ACL; Daniel Ingemansen, Sanford Health; Ericka Olgaard, DO, MBA, University of Arkansas for Medical Sciences; Sterling Bennett, MD, MS, Intermountain; and Judy Lyzak, MD, MBA, Alverno.
Read More »Finding the morphologic clues to neutrophilia etiology
February 2021—Granulocyte morphology may contain clues to neutrophilia etiology, and that was the focus of a CAP20 virtual presentation by Olga Pozdnyakova, MD, PhD, associate professor of pathology at Harvard Medical School and medical director of the hematology laboratory at Brigham and Women’s Hospital. Reactive changes can mimic myeloproliferative neoplasm, but myeloproliferative neoplasm can have reactive morphology, she said. Pathologists can piece together clinical and morphological clues, “especially in concert with the clinical team, that may help them decide whether the changes are more reactive or more neoplastic in nature,” she told CAP TODAY in a follow-up interview. Neutrophilia is defined as greater than 7.7 × 109/L or two standard deviations above the mean, and it is important to note whether it is present in the context of the left shift.
Read More »CAP TODAY Roundtable: AP computer system— ‘Look at value versus cost’
February 2021—What is the one most important thing to look for in an anatomic pathology computer system? That is one of several questions CAP TODAY publisher Bob McGonnagle put to five people in a Dec. 14 call on the AP LIS and more—surgical pathology volumes amid COVID-19, data integration, practice consolidation.
Read More »Surgical Pathology Review: easing the transitions
February 2021—New from CAP Publications is Surgical Pathology Review, by Daniel D. Mais, MD, associate professor of pathology, Department of Pathology, University of Texas Long School of Medicine, San Antonio. He and 14 other contributors wrote this book to ease the transition through board exams and into practice, Dr. Mais writes in the preface. Here is what he told CAP TODAY about the book.
Read More »Practice beyond the microscope, a memoir: My years as a doctor’s doctor
February 2021—When I entered the practice of pathology the role was described as being a “doctor’s doctor.” That reflected the fact that physicians turn to the laboratory for so many tests that define diseases and determine the response to treatment, a reality true today. But my memories are of the more personal interactions with clinicians over the years as I pursued the practice of pathology beyond the microscope, and often beyond the laboratory.
Read More »From the President’s Desk: Important wins but work continues
February 2021—In late December, the U.S. Congress passed a COVID-19 relief bill that included two key items for pathologists—measures that the CAP pushed hard to achieve on our behalf. The document is more than 5,000 pages and addresses a large number of subjects, including thoroughbred horses. Here, I’d like to offer a brief analysis of the elements that affect our profession.
Read More »Q&A column
Q. Can toxicology testing be performed on a person who has been deceased for two years? Read answer. Q. Is there a standardized procedure for performing platelet estimates that incorporates the dilution effect for low hemoglobin in anemic patients? The formula I found for platelet estimation works well with low hemoglobin levels but not with levels greater than 13 g/dL. Read answer.
Read More »Putting labs front, center in pandemic plans
January 2021—Susan Butler-Wu, PhD, D(ABMM), is clear about who she is and what she does. “I’m just a microbiologist,” she says. But in a viral pandemic, a microbiologist—and everyone else associated with clinical laboratory testing—becomes so much more than the job title. (For the record, Dr. Butler-Wu is director of the clinical microbiology laboratory, LAC+USC Medical Center, Los Angeles, and associate professor of clinical pathology, Keck School of Medicine of USC.) Likewise, a test becomes more than a lab value. The very fact that testing has become the focus of national discourse is a testament to the upending nature of the pandemic, she says. “The public are having conversations about Ct values. It’s mind-blowing.”
Read More »Primary HPV screen only? Experts warn of risks
January 2021—It’s a watershed moment when an influential standard-setting organization, the American Cancer Society, announces that a test widely used for decades should be supplanted—especially when the test is the linchpin of the most successful cancer screening program in U.S. history.
Read More »For POC molecular, pauses, plans, and testing precautions
January 2021—The use of molecular assays at the point of care is exciting but a bit scary. That’s how Raquel Martinez, PhD, D(ABMM), described the state of the science for molecular infectious disease POC testing when she spoke in a virtual AMP session in November with Omai Garner, PhD, D(ABMM), of UCLA Health.
Read More »No letup in pandemic struggle for supplies, staff
January 2021—Wrestling with rapid test shortfalls and with open laboratory positions. Expecting the post-holiday surge, frustrated by federal level disconnect. That is what Compass Group members were doing and feeling on Dec. 1 last year, when they gathered on Zoom for another conversation about COVID-19.
Read More »In memoriam: Mary E. Fowkes, MD, PhD
January 2021—Mary E. Fowkes, MD, PhD, a member of the CAP Board of Governors since 2019, died on Nov. 15, 2020. Dr. Fowkes was an associate professor of pathology and the director of neuropathology and the autopsy service, Icahn School of Medicine at Mount Sinai Hospital. She was also an adjunct professor at the State University of New York College of Environmental Science and Forestry in Syracuse.
Read More »Practice management network: A new ‘safety zone’ for practice problem-solving
January 2021—A CAP Practice Management Network will be online Feb. 9 for CAP members and their practice staff who want to share problems and best practices and learn from the practice management insights of others. It will be a “no-judgment zone, a safety zone, a neutral territory,” says Brian H. Le, MD, MBA, co-coordinator of the network and vice chair of the CAP Practice Management Committee, developer of the network. “A lot of practices and new-in-practice pathologists encounter a competitive spirit that sometimes doesn’t serve us well,” says Dr. Le, a pathologist based at Novant Health in North Carolina.
Read More »Congress mitigates Medicare cuts at 11th hour
January 2021—Hanging over the publication of the 2021 Medicare physician fee schedule on Dec. 1 was an overall decrease of nine percent to pathology services and similar cuts to other specialty physicians. The Centers for Medicare and Medicaid Services in 2019 announced these payment cuts would offset increases to evaluation and management services, which are typically billed during physician office visits. The CAP through its advocacy opposed the cuts to pathologists and sought to stop them from taking effect.
Read More »AMP case report: 18-month-old female with poorly differentiated cerebellar tumor harboring BCOR internal tandem duplication
January 2021—Central nervous system high-grade neuroepithelial tumor with BCOR alteration (CNS HGNET-BCOR) is a rare and genetically distinct CNS neoplasm that occurs predominantly in the pediatric population. Previously, these tumors had been categorized as primitive neuroectodermal tumors of the central nervous system (CNS-PNET).
Read More »Coagulation tests and COVID: inside labs, industry
January 2021—COVID-19 and coagulation testing were up for discussion on Nov. 20 when six people joined CAP TODAY publisher Bob McGonnagle to talk about that and laboratory labor, relationships with industry and hospital administration, and the distribution of testing. “We’re working with all the manufacturers to support rapid point-of-care testing to manage hot spots that will pop up once there is a vaccine,” said Orchard Software’s Curt Johnson. With Johnson and McGonnagle on the call were Oksana Volod, MD, of Cedars-Sinai Medical Center; Neil Harris, MBChB, MD, of the University of Florida; Annie Winkler, MD, MSc, of Instrumentation Laboratory; Nichole Howard, MBA, of Diagnostica Stago; and Jason Lam, MBA, MLS, of Siemens Healthineers. Drs. Volod and Harris are members of the CAP Hemostasis and Thrombosis Committee.
Read More »Cytopathology in focus: Virtual education in cytology: pandemic silver lining
January 2021—Although the challenges we face due to the COVID-19 pandemic are significant, adapting to our new circumstances can be a driver for positive change. Cytology education had started using virtual learning resources in the past few years with great variation among institutions and countries. The pandemic forced the community to embrace virtual learning in a variety of modalities. These changes may lead to lasting improvements in the quality and accessibility of educational opportunities for cytology trainees, and not just in the United States. In the same way, we are witnessing the exponential growth of digital pathology as applied to surgical pathology. In cytopathology the applications have been limited to telecytopathology in the setting of rapid on-site evaluation (ROSE) for interventional procedures. The difficulties in dealing with multilayered focus have been given as reasons not to pursue whole slide imaging for cytology. As the cytology community has, even if reluctantly, gained experience with remote sign-out, we may see a push for digital imaging for primary diagnosis in cytology.
Read More »Cytopathology in focus: Telecytology for rapid on-site evaluation
January 2021—Rapid on-site evaluation (ROSE) for cytology specimens is performed at many institutions to improve the quality of health care by proper triage of obtained material to increase the diagnostic yield, or to direct appropriate investigation. It also helps to control health care costs by reducing the rate of nondiagnostic specimens, unnecessary passes, and repeat procedures. The number of procedures requiring ROSE is growing due to the increase in the number of platforms used to perform minimally invasive procedures.
Read More »Q&A column
Q. If an instrument is moved a short distance, is it necessary to conduct revalidation? Read answer. Q. At what level or time is aPTT considered incorrect? Is an aPTT of less than 22.0 seconds an acceptable result? Read answer.
Read More »Governor positions to open, candidates welcome
January 2021—The CAP election to fill four positions on the Board of Governors will take place this summer. The CAP is encouraging its members who have been CAP fellows for at least five years and involved in CAP volunteer activities to take the next leadership step.
Read More »In SARS-CoV-2, small steps but big wins
December 2020—By its very nature, the global pandemic has forced laboratories to look far and wide, to bring binoculars, in essence, to their views of supply chains, testing platforms, personnel, and the like. As COVID-19 churns on, some labs are looking through a tinier lens as well. These labs aren’t trading their binoculars for a jeweler’s loupe, exactly, but they have found small and significant success stories closer to home. Like so many others, Erin Graf, PhD, D(ABMM), has confronted a spinning roulette wheel since the pandemic’s start. In a talk she gave in an AMP webinar in October, Dr. Graf posted a vibrantly colored wheel titled, “Which supply chain issue will impact us this week?” Each segment contained a phrase familiar to everyone in 2020, ranging from “swabs” and “sheep blood agar” to “pipette tips” and “chlamydia and gonorrhea tests.” As she surveys these continuous claims on her attention, Dr. Graf says, “I think none of us could have ever thought that COVID would have an impact on all these arms of the testing that we do.”
Read More »Digging into drug’s effect on aPTT-based assays
December 2020—As ongoing studies reveal the merits of emicizumab for hemophilia A patients—fewer bleeding episodes, longer duration between treatments—laboratories need to be alert to the drug’s effect on coagulation testing.
Read More »How to right the wrongs of clinical decision support alerts
December 2020—Always think about timing, maintain a log of malfunctions, and make the right decision the easy decision. These are a few of the clinical decision support tips that Ronald Jackups Jr., MD, PhD, and Amanda Blouin, MD, PhD, presented last month in a CAP20 session.
Read More »Journeys to alternative SARS-CoV-2 strategies
December 2020—In Colorado, Joan Coleman, MBA, MT(ASCP), and her UCHealth colleagues launched an expansive pooled testing program this summer that, after much work, worked well—until it didn’t, thanks to rising positivity rates in October.
Read More »Identifying respiratory pathogens: Pneumonia panel studied against standard of care
December 2020—In an evaluation performed at Washington University in St. Louis and published recently, BioFire’s FilmArray pneumonia panel was found to have strong agreement with standard-of-care methods in identifying viral and bacterial targets in 200 lower respiratory tract specimens (Webber DM, et al. J Clin Microbiol. 2020;58[7]:e00343–20). It was also found to have strong agreement with the BioFire upper respiratory panel for common targets, making it unnecessary to perform both. In comparison to standard-of-care methods, it has the potential to detect more Staphylococcus aureus and Haemophilus influenzae and to detect more antimicrobial resistance, particularly at low organism concentrations or in mixed cultures.
Read More »Solving problems, restricting orders: Compass on COVID
December 2020—The Compass Group reconvenes to share the latest on SARS-CoV-2 testing—this time on Oct. 6 and again by Zoom. What they said about supplies, labor, and flu follows. Serology testing too: “It’s the one test we have loads of and the one test they don’t use a lot of,” said Heather Dawson of Allina Health in Minneapolis. CAP TODAY publisher Bob McGonnagle led the roundtable. With Dawson were Walter Henricks, MD, of Cleveland Clinic; Jennifer Laudadio, MD, of the University of Arkansas for Medical Sciences; Joseph Baker of Baylor Scott & White; Judy Lyzak, MD, MBA, of Alverno; Susan Fuhrman, MD, of OhioHealth; Dan Ingemansen and Rochelle Odenbrett, MT(ASCP), MBA, of Sanford Health; Janet Durham, MD, of ACL Laboratories; Diana Kremitske, MS, MHA, MT(ASCP), of Geisinger; Darlene Cloutier, MSM, MT(ASCP), HP, of Baystate; Stan Schofield of NorDx; Clark Day of Indiana University Health; Tylis Chang, MD, of Northwell; and John Waugh, MS, MT(ASCP), of Henry Ford. The Compass Group is an organization of not-for-profit IDN system lab leaders who collaborate to identify and share best practices and strategies.
Read More »Urinalysis: ‘a field with the potential to do more’: pathologist, two companies talk about urinalysis now and what’s needed
December 2020—What could improve urinalysis operations in your laboratory? That’s a question CAP TODAY publisher Bob McGonnagle asked Megan Nakashima, MD, of the Cleveland Clinic when she talked in October with him and two others: Carl Trippiedi of Sysmex and Matt Rhyner, PhD, MBA, of Beckman Coulter. Their conversation took place as CAP TODAY’s 2020 product guide to urinalysis instrumentation was taking shape. What they had to say follows.
Read More »Q&A column
Q. Can a heel stick for a basic metabolic panel with magnesium and phosphorus be performed on a two-month-old baby? Read answer. Q. Due to nationwide supply shortages affecting COVID-19 and other testing in the laboratory, we are concerned about using up critical supplies when assessing competency. Do you have suggestions or strategies we can use? Read answer. Q. When a patient is admitted to our hospital, we collect MRSA nares PCR, MRSA axilla by culture, MRSA groin by culture, and vancomycin-resistant Enterococcus by PCR for infection control purposes. Many surrounding facilities have told us they have removed the axilla and groin cultures, but no references were cited to support removing these procedures. Our facility would like to follow the practices of other hospitals, but our providers would like a reference to cite. Are there best practices or benchmarks from an infection control and microbiology point of view that would allow us to remove the axilla and groin MRSA screen cultures? Read answer.
Read More »Making peace with saliva, pooled testing
November 2020—Adam Barker, PhD, D(ABMM), was ready to call it quits. For weeks, he had been working to bring saliva-based SARS-CoV-2 testing to ARUP Laboratories and the University of Utah. Dr. Barker, director of ARUP’s COVID-19 rapid response lab, and his colleagues had done studies comparing saliva with nasopharyngeal swabs, which seemed to be following the flight of the passenger pigeon out of existence. They had wrestled with the FDA over emergency use authorization. They’d developed their own transport media, since that supply was also becoming extinct. He had begun building kits for saliva collection and figured out what sample size worked best. Kits had been delivered to collection sites on campus, and staff were being trained in their use. He was, in other words, creating a laboratory success story, one of the many that have been written since March. He was not basking in this fact. “I have to tell you: I lost so much sleep because of saliva,” says Dr. Barker, who is also director, ARUP Institute for Clinical and Experimental Pathology.
Read More »Checklist, CLIA line up on COVID reporting
November 2020—It’s been well understood since the Ten Commandments that rules that appear simple in theory can be fiendishly complex or even impossible to execute. The pandemic is providing a perfect example of that in the laboratory world, but with added twists, at least for now.
Read More »Three at AACC: rapid STI testing, toxicology, biosafety
November 2020—Point-of-care testing for sexually transmitted infections, toxicology investigation, and biosafety practices are three of the hundreds of topics that will come online next month during AACC’s virtual annual meeting.
Read More »Fewer urine cultures — series of changes add up
November 2020—Five years after putting in place a urine reflex algorithm at Barnes-Jewish Hospital in St. Louis, and many tweaks later, Melanie Yarbrough, PhD, D(ABMM), D(ABCC), has tips to share on how to increase the odds for success in reducing the number of urine cultures.
Read More »At POC and in lab, 2 new checks on SARS-CoV-2 testing
November 2020—The CAP released in September its proficiency testing program for SARS-CoV-2 antigen testing, with the first shipment to laboratories set for Nov. 30. It also introduced recently a Quality Cross Check program that makes it possible for labs performing nucleic acid amplification testing for SARS-CoV-2 to monitor performance across multiple instruments, in compliance with the CMS directive prohibiting proficiency testing on multiple instruments.
Read More »Compass on COVID: What test for whom and when—lab leaders talk
November 2020—Testing saliva, stocking up, and expanding capacity were top of mind when members of the Compass Group convened by Zoom on Sept. 1 for a second COVID-19-related call with CAP TODAY publisher Bob McGonnagle. Antigen testing, too, came up, and the question to answer there, said Susan Fuhrman, MD, of OhioHealth, is why the test is performed and what will be done with the result. That and more—testing for patients undergoing treatment for cancer, flu season—were up for discussion. Others on the call were Greg Sossaman, MD, of Ochsner; Lauren Anthony, MD, and Heather Dawson of Allina; Sarah Province and Julie Hess of AdventHealth; James Crawford, MD, PhD, of Northwell; Stan Schofield and Robert Carlson, MD, of MaineHealth; Sterling Bennett, MD, MS, of Intermountain; John Carey, MD, of Henry Ford; and Pamela Murphy, PhD, APRN, of MUSC Health. The Compass Group is an organization of not-for-profit IDN system lab leaders who collaborate to identify and share best practices and strategies. (For our coverage of their first call with CAP TODAY, see “Compass points chart the pandemic,” September 2020.) Here is what they told us on Sept. 1.
Read More »IT in a pandemic year, now and what’s ahead: interfaces, analytics, telepathology—seven weigh in
November 2020—Information technology from a COVID-19 perspective. What has been the impact on IT, and what change is yet to come? That is what seven people who met virtually on Sept. 10 talked about with CAP TODAY publisher Bob McGonnagle. They are James Harrison, MD, PhD, of the University of Virginia; J. Mark Tuthill, MD, of Henry Ford; Stephen Hewitt, MD, PhD, of the National Cancer Institute; Bob Dowd of NovoPath; Michelle Del Guercio of Sunquest; Curt Johnson of Orchard; and Brian Gunderson of Roche. You will see here, in the conversation that follows, where their focus is as the crisis continues.
Read More »AMP case report: Role of lymphoma sequencing panel in diagnosis of pediatric-type follicular lymphoma
November 2020—Pediatric-type follicular lymphoma (PTFL) is a rare form of lymphoma that was recognized as a new diagnostic entity in the revised 2016 WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. The classic features of PTFL include male predominance, localized stage I lymphadenopathy, blastoid morphology, high proliferation index, and exceedingly good response rate to local excision.
Read More »From the President’s Desk: Gratitude all around
November 2020—Despite the circumstances that forced us to shift to a virtual event for our annual meeting this year—and my sincere hope we will never have to do so again—I couldn’t be more proud of how everyone in the CAP worked hard to adapt to the COVID-19 pandemic and ensure the best experience possible for all of our pathologists. As I write this, the annual meeting has just concluded. While every conference we hold requires tremendous effort, this was a different event, a different horse race. The work and persistence that went into making our virtual meeting a success were extraordinary.
Read More »Q&A column
Q. What can laboratories expect to see after a medication such as Narcan is given for an opioid overdose? Read answer. Q. There are conflicting views among my colleagues regarding the meaning of initial competency assessment. Some think that using a training checklist for new staff counts as the initial competency assessment because we are signing off that staff are competent to perform patient testing and report results. Others believe an initial competency assessment is done shortly after training is completed, followed by the mid-cycle/six-month competency assessment and annual competency assessment. Please clarify. Read answer. Q. How do you calculate RDW-SD and RDW-CV values in dimorphic anemia cases on the Sysmex XN-3000? Most of the dimorphic anemia cases report a masked parameter. Read answer.
Read More »Flu mounts COVID’s bustling stage
October 2020—Barely a half year into the pandemic’s presence in the United States, history has already begun pressing down on SARS-CoV-2 testing. Like an actor playing Hamlet, it’s been difficult not to feel the burden of past performances when preparing for the months ahead. Now, at the start of fall, that also means readying for the return of influenza. Here, even longer experience has shown that each new season is, indeed, a new season. As in the theater world itself these days, planning for what lies ahead feels tempest-tossed. Plans are being laid. Discussions continue. Creativity abounds, and hard work persists. The season shall unfold. But no one knows how it will look until the curtain—or whatever is passing for one this year—goes up. Poor Hamlet is troubled enough to fill the stage for hours—it is, in fact, Shakespeare’s longest play. Yet he’s just one man. Laboratories this fall are absorbing the slings and arrows of two roles simultaneously. Can they prepare for both parts (think Richard II and III sparring on the same stage) with confidence?
Read More »LSU autopsy findings point to endothelium as target in heart
October 2020—Autopsies conducted at University Medical Center in New Orleans on 22 patients who died from SARS-CoV-2 infection found not the expected typical inflammation of the heart muscle associated with myocarditis but instead scattered individual myocyte necrosis.
Read More »Higher CVD risk, or lower risk? hs-cTn in diabetes
October 2020—When Elizabeth Selvin, PhD, MPH, of Johns Hopkins Bloomberg School of Public Health, began her studies of high-sensitivity cardiac troponin assays, they had not yet been approved in the U.S., as they are now, for use in diagnosing myocardial infarction. But some of her studies and those of Amy K. Saenger, PhD, DABCC, medical director of clinical laboratories and director of clinical chemistry at Hennepin County Medical Center in Minneapolis, take high-sensitivity cardiac troponin in a new direction by exploring its potential use as an aid in monitoring cardiovascular risk in the general population.
Read More »Uncharted season forges new paths for all hands
October 2020—Planning for respiratory season is always tricky but never more so than this year. “Uncharted territory for influenza” is how Frederick Nolte, PhD, D(ABMM), of the Medical University of South Carolina, describes the prospect of testing for influenza at the scale labs have been testing for SARS-CoV-2.
Read More »Molecular methods shown to push cases forward: Case studies in hematopathology
October 2020—B-ALL with aberrant expression of myeloid markers should be investigated further for specific gene abnormalities, including ZNF384 rearrangements, and microarray analysis may play an important role.
Read More »AMP case report: TET2TET— reconciling conflicting genomic reports
October 2020—After 20 years of CAP advocacy, synoptic reporting in surgical pathology is ubiquitous. This came about in part by fiat and in part by all parties agreeing on the importance of standardization for patient care. The merits of some elements remain controversial. Molecular pathology, a newer discipline, does not offer the scope for creative writing once available in surgical pathology.
Read More »MGMT promoter methylation: assays, implications
October 2020—With MGMT gene promoter methylation observed in about 50 percent of glioblastomas, it remains a biomarker of strong clinical interest in routine practice, even though it’s not the sole determinant in decisions related to therapy. PCR and pyrosequencing are the most commonly used assays, and there’s a technique that is not yet mainstream but gaining interest, said Tejus A. Bale, MD, PhD, assistant attending pathologist in the Department of Neuropathology and Diagnostic Molecular Pathology, Memorial Sloan Kettering Cancer Center. Dr. Bale spoke June 30 in the first of a series of Association for Molecular Pathology webinars on emerging and evolving biomarkers.
Read More »From the President’s Desk: An access to care issue
October 2020—Many CAP TODAY readers know about my fondness for horse racing, so it will come as no surprise that I thoroughly enjoyed the belated running of the Kentucky Derby last month. I even made a few dollars from a small bet, which may be the only positive financial news I get in a year marked by COVID-19 and the threat of impending reimbursement cuts.
Read More »Compass points chart the pandemic
September 2020—Between a rock and a hard place. Trying to stay ahead, trying to build inventory. Chasing multiple new testing requests. Anticipating influenza. That’s where laboratory leaders said their labs were in early August when CAP TODAY publisher Bob McGonnagle convened members of the Compass Group on Zoom to share their pandemic experiences. They shared surprise, too, that the situation is what it is: “Not a clue in my mind that this would go past the springtime,” said Stan Schofield, president of NorDx and senior VP, MaineHealth. McGonnagle asked them about the diversion of supplies, the coming flu season, IT support, lessons and long-term changes, and more.
See current issue below for additional COVID-19 coverage or access all COVID-19 articles here.
Real-time QC: on course for prime time?
September 2020—Bill Gates was just 10 years old and the Beatles were still playing live concerts when the concept of patient-based real-time quality control was proposed in 1965. At the time, patient-based real-time QC (PBRTQC) was based on the “average of normals,” a precursor of moving averages.
Read More »Oh, the places you’ll go when flu season hits
September 2020—The twinned challenge of testing for SARS-CoV-2 and the upcoming influenza season has a bit of The Cat in the Hat energy running through it. How does one manage to keep Thing One and Thing Two from creating unmitigated chaos? Maybe one doesn’t, not completely. A pandemic-based flu season will by its very nature be protean.
Read More »What’s new in latest transfusion medicine checklist
September 2020—Strong quality management, patient safety, and conformity with regulations are at the heart of the new and revised requirements in the 2020 CAP accreditation program transfusion medicine checklist, released in June.
Read More »Juan Rosai, ‘master of the neoplastic universe’
September 2020—Great mentors, once passed, live on in the ideas, memories, and dreams of their students. For those like me, lucky enough to have been a student of Juan Rosai, MD, and to have heard him speak, the experience will never be forgotten.
Read More »Why do universal HRD testing in ovarian cancer?
September 2020—Genetic testing in ovarian cancer has a therapeutic implication that will aid in developing a treatment plan, and it is pathologists who should take the lead in creating the testing protocol, said Samuel Caughron, MD, pathologist, president, and CEO of MAWD Pathology Group, in a recent CAP TODAY webinar. Dr. Caughron explained the rationale for universal homologous recombination deficiency testing in patients with advanced ovarian cancer. The webinar, made possible by a medical sponsorship from AstraZeneca, is at <a href="https://www.captodayonline.com">www.captodayonline.com</a>.
Read More »Behind book on professionalism: ‘we can do better’
September 2020—Professionalism in Pathology and Laboratory Medicine is a new book now out from CAP Publications. It provides a basic understanding, educational and assessment tools, 105 cases specific to pathology and laboratory medicine, guidance in recognizing and addressing lapses in behavior, discussions on best practices and legal and ethical aspects, and much more. Ronald E. Domen, MD, of Penn State College of Medicine and Hershey Medical Center, is editor. His co-editors are Richard M. Conran, MD, PhD, JD, of Eastern Virginia Medical School; Robert D. Hoffman, MD, PhD, of Vanderbilt University School of Medicine; Cindy B. McCloskey, MD, of the University of Oklahoma Health Sciences Center; and Suzanne Zein-Eldin Powell, MD, of Houston Methodist Hospital.
Read More »Targeting immune signaling checkpoints in AML
September 2020—Acute myeloid leukemia was one of the first diseases for which T cells were incorporated into the therapeutic paradigm, in the form of allogeneic stem cell transplant and donor lymphocyte infusion. Why then are there no approved immune therapies, or more specifically checkpoint inhibitors, for this T-cell–sensitive disease?
Read More »AMP case report: Culture-negative endocarditis due to Tropheryma whipplei
September 2020—A 64-year-old male presented with worsening shortness of breath, dry cough, and bilateral leg edema. He had a history of diabetes mellitus type two, hypertension, seropositive rheumatoid arthritis, and tobacco and alcohol abuse. CT scan demonstrated bilateral pleural effusions, pulmonary edema, subsegmental atelectasis, mildly enlarged hilar lymph nodes, mild cardiomegaly with a small pericardial effusion, and liver cirrhosis with a liver nodule. A hepatitis panel demonstrated positive serology for hepatitis C virus infection.
Read More »The laboratory tests of pandemic summer
August 2020—In March, the COVID-19 pandemic came in like a lion—and has yet to leave, like a lamb or anything else. Instead, it roared through April and May in early hot spots like New York City and New Orleans. As lockdowns took hold, the cautious hope was that by summer the virus would be tamed (if not simply go away “like a miracle” or “as the heat comes in,” per several infamous predictions), giving health care providers a chance to exhale before a likely second wave in the fall. Instead, June and July saw other cities and states hit hard in turn, while many places that appeared to have flattened the curve were starting to see concerning upticks in cases.
Read More »Targeting microbiology lab efficiency with AI
August 2020—Bringing an automated culture plate reading system into the Hennepin County Medical Center microbiology laboratory was never a question of if but when. “We need artificial intelligence to help us with active decision-making processes in the lab,” says Glen Hansen, PhD.
Read More »In 2020 checklist, a ‘gentle push’ to next quality level
August 2020—For quality management in the laboratory, it’s not enough to have checks and balances. The checks and balances have to work to improve quality. That’s how Stephen J. Sarewitz, MD, vice chair of the CAP Checklists Committee, characterizes the changes to the quality management requirements in the 2020 laboratory general checklist, released in June.
Read More »Lab with Ebola experience: COVID more complicated
August 2020—If there’s one thing scarier to experience than COVID-19, it’s Ebola. Or so you might think. “Ebola was easier,” says Beverly Dickson, MD, medical director of the clinical laboratory at Texas Health Presbyterian Hospital Dallas.
Read More »Steps to verifying SARS-CoV-2 antibody assays and what’s known about protective immunity
August 2020—The CAP treats emergency use authorization assays similar to FDA-cleared assays and thus requires full verification. In a June 4 CAP webinar, Neil Anderson, MD, D(ABMM), assistant director of clinical microbiology, Washington University School of Medicine in St. Louis, walked through how to approach verification for SARS-CoV-2 assays. Co-presenter Elitza Theel, PhD, D(ABMM), director of the infectious diseases serology laboratory at Mayo Clinic, reported what’s known about protective immunity against SARS-CoV-2.
Read More »Tumor budding assessment in CRC: why and how
August 2020—Tumor budding is a robust prognostic marker that should be reported at least in pT1 and stage II colorectal carcinomas and taken into account with other risk factors. Further evidence is needed for tumor budding assessment in specimens taken after neoadjuvant therapy, says Heather Dawson, MD, senior staff GI pathologist at the Institute of Pathology, University of Bern in Switzerland.
Read More »AMP case report: Burkitt-like lymphoma with 11q aberration
August 2020—Burkitt-like lymphoma with 11q aberration (BLL-11q) is a new provisional entity in the revised 2016 WHO classification of hematopoietic and lymphoid tumors.1 It refers to a subset of high-grade B-cell lymphomas that resemble Burkitt lymphoma with similar morphology, phenotype, and gene expression profiling, but lack MYC gene rearrangements. Instead, these lymphomas carry chromosome 11q proximal gains and telomeric losses, suggesting co-dysregulation of oncogenes and tumor suppressor genes.
Read More »COVID testing capacity falls short as flu season nears
August 2020—As the need for COVID-19 testing grows well beyond that for hospital patients, clinical laboratories in mid-summer were again overwhelmed by demand while at the same time bracing for flu season. That was the gist of a July 10 webinar that brought together Gyorgy Abel, MD, PhD, medical director of clinical chemistry, molecular diagnostics, immunology, and point-of-care testing at Lahey Hospital and Medical Center, Burlington, Mass.; Bob McGonnagle, CAP TODAY publisher; and moderator Steve Beuchaw, director of life science and medical device research, Wolfe Research.
Read More »Cytopathology in focus: Abnormal cervical screening tests: a personal approach
August 2020—If the past decade was directed toward aligning medicine with a personalized approach to therapy, this decade should further realize the implementation of health care decisions tailored to the patient. The updated 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors take a large step in that direction by relying on the input of personal data into a free online application that provides suggested management planning based on patient history and prior Pap/HPV test results.
Read More »Cytopathology in focus: New direction for thoracic small biopsy, cytology specimens
August 2020—Cytopathologists are keenly aware of the need to collect adequate cytologic tissue not only to arrive at a diagnosis but also to provide sufficient material for predictive and prognostic markers. This is especially true in the realm of non-small cell lung cancer, where biomarker testing is routinely used for the clinical management of patients with advanced-stage disease. The list of clinically relevant biomarkers in NSCLC is expanding. The most recent version of the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology includes MET exon 14 skipping mutations and RET as therapeutic targets for advanced NSCLC, in addition to the well-established EGFR and BRAF mutations, ALK and ROS1 rearrangements, and PD-L1 expression.
Read More »A preanalytical Rx for fallible GDM testing
July 2020—Gestational diabetes mellitus, if left untreated, is notoriously dangerous for mothers and their babies, making timely diagnosis critical. Yet the disease is similarly well known for being chronically under-diagnosed by laboratory testing.
Read More »New checklist hones lab’s verification and validation requirements
July 2020—If a manufacturer assists a laboratory in setting up a new FDA-approved or -cleared test, the lab must make sure that the personnel who will perform the test participate in the verification or validation study.
Read More »Outbreak detection of novel pathogens: Is AI the answer?
July 2020—A machine learning algorithm, used in conjunction with BioFire’s Syndromic Trends, demonstrated a mechanism for near real-time outbreak detection of enterovirus D68, says a study reported in the Journal of Clinical Virology.
Read More »In CRC, distinguishing tumor deposit from lymph node
July 2020—When patients who have colorectal cancer surgery at another institution seek further care at Beth Israel Deaconess Medical Center in Boston, the Beth Israel pathologists routinely request the original slides. Raul S. Gonzalez, MD, a gastrointestinal pathologist at Beth Israel, says he usually agrees with everything the outside pathologist reports. But if there are differences, lymph nodes versus tumor deposits is one place where he might disagree.
Read More »Closing the workflow loop: HistoQC for digital slides
July 2020—Unveiled in 2018, HistoQC, an open-source quality control tool for digital pathology slides, was an “awakening to a problem” and the kickoff of a conversation, says Andrew Janowczyk, PhD, its main investigator. And while it’s hard to measure the tool’s use because it’s freely available, he says, reception has been strong. “It’s been a pretty good ride,” he says of its first two years.
Read More »AMP case report: Unexpected diagnosis of indolent systemic mastocytosis through evaluation of NGS data
July 2020—A 77-year-old woman presented at our institution for care with a remote history of lymphoma. Three years earlier she had a bone marrow biopsy performed that by report showed five percent monoclonal kappa restricted plasma cells. At the initial presentation at our institution, serum protein electrophoresis and immunofixation showed an IgA kappa paraprotein (0.52 g/dL), and bone marrow biopsy showed less than five percent plasma cells by CD138 immunohistochemistry that were kappa light chain restricted by flow cytometry, with normal renal function and calcium level. A PET-CT indicated no lytic bone lesions. These findings confirmed the diagnosis of IgA kappa monoclonal gammopathy of undetermined significance (MGUS). Her past medical history was remarkable for chronic oral ulcers for five years of possible autoimmune etiology that were responsive to methylprednisolone, a stroke, hypertension, and hyperlipidemia.
Read More »At UW, anatomic pathology rotation moves online
July—When COVID-19 set in, much of residency education in the U.S. moved online. At the University of Washington School of Medicine, anatomic pathology faculty took online learning a step further by creating a virtual two-week anatomic pathology rotation for medical students. The faculty is aiming for a four-week virtual rotation inclusive of more laboratory medicine, to be used even after the pandemic has passed.
Read More »A panel’s take on instruments, connectivity, COVID
July 2020—Has the pandemic changed your thinking or that of your customers? That’s one of the questions CAP TODAY publisher Bob McGonnagle put to seven representatives of five companies and two other panelists in a May 13 roundtable on chemistry/immunoassay analyzers and testing. But first up were other topics: scalability, connectivity, standardizing platforms across health systems, consistent sourcing of antibodies, and open automation.
From the President’s Desk: Remembering Gene Herbek
July 2020—It is with profound sadness that I write about the passing of a dear friend. Gene Herbek, MD, served as president of the CAP from 2013 to 2015, and many CAP TODAY readers will remember his informative column in these pages. For details about his life and accomplishments, please read his obituary in this issue (page 12). I’d like to spend this column focused on how he shaped the CAP, and what an honor it was to have known him.
Read More »In memoriam: Gene N. Herbek, MD 1949 –2020
July 2020— Gene N. Herbek, MD, CAP president from 2013 to 2015, died June 4 after a brief illness. He was a CAP governor from 1998 to 2004 and secretary-treasurer from 2006 to 2011. Dr. Herbek was medical director of the Methodist Women’s Hospital laboratory and medical director of transfusion services for The Pathology Center at Methodist Hospital, Omaha, Neb. He chaired multiple CAP groups over the years, among them the councils on Public Affairs and Membership and Public Affairs and the Finance, Credentials, Nominating, Political Action, and Insurance committees.
Read More »At the pandemic’s serologic frontier
June 2020—The arrival of a pandemic has shown—among many, many other things—that anyone who talks about it typically starts by saying, “This is a pandemic.” The next sentence tends to be, “It’s a completely different situation,” whether the focus is grocery shopping, exercising (or not), voting, or practicing medicine. Pointing to the pandemic is a polite way of saying, “All bets are off.” For many, it’s been a springboard to innovation and breakthroughs, even in the midst of considerable anguish. For clinical laboratories, however, much has felt unsettling, especially when the conversation turns to serology testing for SARS-CoV-2. It’s a topic stuffed to overflowing with interest, enthusiasm—and, early on, antibody tests themselves.
Read More »Published in June: Autopsies show many faces of COVID-19 Sudden” and “global” are descriptors that seldom appear in tandem, especially in relation to disease epidemiology. But they both fit the COVID-19 pandemic, which has left the health care world reeling. “COVID-19 has infected the entire planet pretty much all at once,” says Alex K. Williamson, MD. Read more.
Read More »HIV, TB requirements in latest accreditation checklist edition
June 2020—Best practices for HIV primary diagnostic testing and rapid detection of Mycobacterium tuberculosis complex are clarified and codified in new checklist requirements in the 2020 Laboratory Accreditation Program checklist edition published June 4.
Read More »ER, PgR, HER2 expression rates seen in Q-Probes
June 2020—With release of the latest Q-Probes study, titled “Expression Rates in Invasive Breast Carcinoma,” the CAP Quality Practices Committee fills a gap by providing data collected from a diverse set of 21 U.S. laboratories on the average frequency of various ER, PgR, and HER2 expression results.
Read More »Amid COVID-19 crisis, pathologists fill a critical gap
June 2020—At NYU Langone Health, pathologists and others typically not seen out front in the fight against COVID-19 became the bridge between families and the floors. When Katherine A. Hochman, MD, associate chair for quality in the Department of Medicine at NYU Langone, contracted a mild case of COVID-19, she finally had a chance to take a step back and think. Before going into quarantine to recover, Dr. Hochman had been on the floors day in and day out attending to COVID-19 patients.
Read More »Predicting response to therapy with BH3 profiling
June 2020—Precision medicine in oncology, which today is nearly universally about genetics, needs to move beyond omics and static approaches, Anthony Letai, MD, PhD, professor of medicine at Dana-Farber Cancer Institute and Harvard Medical School, said at last year’s meeting of the Association for Molecular Pathology. Dr. Letai reported how his laboratory uses dynamic BH3 profiling, a novel assay that detects BCL2 protein dependence in cancer cells and measures changes in their apoptotic priming, to predict clinical response to therapy. He and others call it functional precision medicine.
Read More »In NSCLC, biomarker testing rates fall short
June 2020—Testing rates for actionable biomarkers in metastatic non-small cell lung cancer patients are below where they should be, and the overlap of PD-L1 expression with genomic targets causes confusion for oncologists and patients, said Geoffrey R. Oxnard, MD, oncologist at Dana-Farber Cancer Institute and associate professor of medicine at Harvard Medical School, in a recent CAP TODAY webinar.
Read More »AMP case report: CCND1/IGH fusion amplification in a case of plasma cell myeloma
June 2020—A middle-aged adult presented with shortness of breath and bruising and was found to have leukopenia (WBC 4.16 K/mcL; normal range 4.50–11.00 K/mcL) and anemia (Hgb 7.6 g/dL; normal range 12–16 g/dL) with rouleaux. The hypercellular bone marrow core biopsy (80–90 percent cellularity) contained 90 percent plasma cells of variable morphology, some with prominent nucleoli and occasional binucleate forms.
Read More »In Italy, lessons learned for lab testing
June 2020—The key lesson for policymakers and hospital administrators stemming from the pandemic is that continuing to cut human and economic resources will create large organizational issues when the entire system of care, including laboratory diagnostics, is challenged by “an enormously amplified volume of tests to manage emergent situations,” write Giuseppe Lippi, MD, of the University of Verona, and Mario Plebani, MD, of University Hospital of Padova, Italy, in an opinion paper published online March 19.
Read More »Letters
June 2020—I enjoyed the recent roundtable on billing and reimbursement (April 2020), along with the product guide on billing software. As we all know, since that time COVID-19 has taken center stage. Here are some thoughts and predictions to bring your readers up to date. Never before has a nation turned off a large percentage of its health care system overnight. This change has caused a train wreck-like boxcar effect that is rippling through medical billing operations for laboratories and pathology groups.
Read More »Published in July:
Reading COVID-19’s signature: lung tissue injury
 Alain Charles Borczuk, MD, began his practice of pathology as a resident 28 years ago and has spent quite a bit of his career doing autopsies. But it is this year, during the pandemic, that he’s finding some of the best applications of his autopsy work as he seeks to understand the lung injury patterns in SARS CoV-2, or COVID-19 patients. Read more.
Alain Charles Borczuk, MD, began his practice of pathology as a resident 28 years ago and has spent quite a bit of his career doing autopsies. But it is this year, during the pandemic, that he’s finding some of the best applications of his autopsy work as he seeks to understand the lung injury patterns in SARS CoV-2, or COVID-19 patients. Read more.
A lab world embroiled in pandemic
May 2020—Along with SARS-CoV-2, clinical laboratory testing has been hiding in plain sight far longer than many people realize. But it took the novel coronavirus (which, frankly, hardly feels novel anymore) to make that clear to the rest of the world. As the COVID-19 pandemic spread across the globe, laboratory testing crashed the news cycle. National leaders sought to reassure citizens by promising millions of test kits.
Read More »Adapting test ordering to novel tick-borne disease rise
May 2020—When tick-borne infections are the topic of discussion, talk of Lyme disease, caused by Borrelia burgdorferi, has tended to predominate. But as the prevalence of tick-borne infections in the United States rises steadily and widens geographically, the number of novel pathogens carried by ticks has been climbing as well.
Read More »Suiting up for COVID-19 autopsies, sharing findings
May 2020—To combat the spread of COVID-19, Louisiana’s governor in late March issued an order directing state residents to limit their excursions outside.
Read More »AI roundtable: hopes, hurdles, hype vs. reality
Michael Becich, MD, PhD, of the University of Pittsburgh School of Medicine, and five others spoke with CAP TODAY publisher Bob McGonnagle in March about the hype versus reality of artificial intelligence and the tension around it. Here is what they had to say.
A conversation: Specimen collection and testing for SARS-CoV-2
May 2020—But as the prevalence of tick-borne infections in the United States rises steadily and widens geographically, the number of novel pathogens carried by ticks—including non-Lyme bacteria, protozoa, and viruses—has been climbing as well.
Read More »In memoriam: William B. Zeiler, MD 1921–2020
May 2020—William B. Zeiler, MD, CAP president from 1987 to 1989, died March 24 at age 99. Dr. Zeiler was CAP vice president from 1985 to 1987 and a member of the Board of Governors from 1981 to 1985. He led and was a member of several CAP councils, committees, and commissions. He was named CAP Pathologist of the Year in 1990, and winner of the Gold Headed Cane Award from the World Association of Societies of Pathology and Laboratory Medicine in 2001.
Read More »From single to syndromic tests for SARS-CoV-2
May 2020—Christine C. Ginocchio, PhD, MT(ASCP), in an April 8 CAP TODAY webinar, reported findings on the analytical efficiencies and sensitivities of four of the original and most common of the SARS-CoV-2 assays. Read more.
Read More »AMP case report: NGS as the tiebreaker in tumors with similar morphology and equivocal immunophenotype
May 2020—Traditionally, histopathologic diagnosis has been regarded as the gold standard for most disease processes including cancer. However, in certain circumstances, a final histopathologic diagnosis cannot be rendered despite extensive conventional ancillary testing such as immunohistochemistry. In recent years, molecular testing has revealed specific variant signatures for many tumors, which can be used to determine a final diagnosis.
Read More »Study of inpatient test utilization practices set to begin
May 2020—Like a top 40 radio hit, test utilization is a topic that can sometimes seem to be overplayed. But the COVID-19 pandemic brings into sharp relief its importance. “What we’ve seen is organizations that have more mature test utilization efforts in place may be better able to handle these crises,” says Peter L. Perrotta, MD, professor and chair of pathology, anatomy, and laboratory medicine at West Virginia University School of Medicine and director of pathology services, West Virginia University Health System.
Read More »Cytopathology in focus: Direct HPV testing in FNAs from cervical lymph node metastases
May 2020—According to a Centers for Disease Control and Prevention study from 2008 to 2012, there are about 16,000 cases of HPV-positive oropharyngeal squamous cell carcinoma per year in the United States. These carcinomas tend to present with small primary lesion and early nodal metastases as an initial manifestation of the disease. Furthermore, carcinoma of unknown primary presenting as a cystic metastasis in the head and neck has been linked frequently to oropharyngeal squamous cell carcinomas, mainly of palatine tonsils and base of the tongue.
Read More »Cytopathology in focus: Gynecologic cytology PT appeals: where they started,where they stand
May 2020—The CAP implemented proficiency testing for cervical cytology in 2006 as mandated by federal legislation. The performance of participants and granting of appeals on glass slides in the first year of the CAP Pap PT program was reported in detail by Crothers, et al. Once a participant initiates an appeal, the slide in question is pulled from the program for a blinded review by three board-certified anatomic pathologists who are members of the CAP Cytopathology Committee. In the first year, 155 participants failed the PT examination and appealed their testing results on 86 individual slides. After review, appeals were granted for 21 slides, resulting in 45 exam failure reversals. The overall appeal rate was 13/1,000 slides in the program.
Read More »Shorts on Standards: ISO 22367: Application of risk management to medical laboratories
May 2020—Hazard. Harm. Risk. Benefit. Performing any activity poses risks. The laboratory deals with hazards known and understood, or unknown and unanticipated, that can lead to harm. Laboratorians attempt to minimize these risks to the point where benefit exceeds residual risk that cannot be further minimized. We understand these concepts and terms and deal with them in everything we do. For optimal patient care and for the safety of our employees, a formal approach to risk assessment is desirable to identify pertinent risks, assess those risks, determine if and when the risk has been sufficiently minimized or if mitigation measures are worth the benefit we expect to see, and document each step.
Read More »AST breakpoints: a case of not aging gracefully
April 2020—When Romney Humphries, PhD, D(ABMM), was section chief for clinical microbiology at UCLA Medical Center, it wasn’t unusual for her to take calls from worried clinicians who were concerned about patients who’d been transferred to the hospital for a higher level of care. The accompanying laboratory reports would indicate the presence of an isolate that was susceptible to a particular drug. But when Dr. Humphries’ lab did its own testing, the results were strikingly different: The isolate was drug resistant. When Dr. Humphries would call the first lab, the reason for the discrepancy became clear. “Sure enough, they were using old breakpoints,” says Dr. Humphries, who is now chief scientific officer, Accelerate Diagnostics, and professor of pathology, University of Arizona. Unlike Bordeaux, antimicrobial susceptibility test breakpoints do not age well. Over time, they might no longer be clinically useful, even as they continue to be used clinically. Dr. Humphries’ anecdote is, of course, just that—an anecdote.
Read More »ER/PgR guideline hones approach to ER-low positives
April 2020—The CAP and the American Society of Clinical Oncology released two years ago a focused update of their clinical practice guideline for HER2 testing for breast cancer, following an update in 2013. Read more.
Read More »Moving beyond immunoassays for poisoned patients
April 2020—Bypassing immunoassays as the first step in toxicology testing can minimize clinician calls to the laboratory about negative toxicology reports for apparently overdosed patients and save diagnostic time. Read more.
Read More »Transfusion cases out of cabinet, into new book
April 2020—CAP Publications released this month a new book titled Transfusion Medicine: A Compendium of Educational Cases, from the CAP Transfusion, Apheresis, and Cellular Therapy Committee. In it are 20 cases, each with a history, discussion, and questions and answers. Read more.
Read More »Billing practices, problems — we ask the experts
April 2020—Billing and collecting for pathology and laboratory services is “tough and getting tougher.” That’s the view of consultant Al Sirmon of Pathology Practice Advisors, Pawleys Island, SC. “In what other industry,” he asks, “do you rely on a third party to decide how much and when you’re going to get paid for a service you provide to someone else?” On the bright side, he says: “We have better tools now.”
PD-L1 testing in triple-negative breast cancer: Post hoc IMpassion130 substudy evaluates PD-L1 IHC assay performance
April 2020—IMpassion130 was the first phase three trial to demonstrate a clinical benefit of cancer immunotherapy in patients with PD-L1-positive metastatic triple-negative breast cancer, and based on the data, atezolizumab plus nab-paclitaxel is approved for this indication. In the trial, the Ventana SP142 PD-L1 assay with a one percent or greater cutoff was used to evaluate PD-L1 expression in immune cells. But questions remained about how to best identify patients who could benefit from the drug combination, Hope S. Rugo, MD, professor of medicine and director of breast oncology and clinical trials education at the University of California San Francisco Comprehensive Cancer Center, said in a CAP TODAY webinar last November.
Read More »Student fellowship’s pluses seen in the field and out
April 2020—At Oregon Health and Science University, one of the oldest post-sophomore fellowship programs in pathology is recruiting steadily and channeling a quarter to a half of its student fellows into pathology residencies. Dating back to the 1920s, the OHSU Pathology Student Fellowship, as it is now known, starts recruiting at the end of the first year of medical school, says Nicole Andeen, MD, assistant professor of pathology and co-director of the program. “Medical students join after the preclinical curriculum or after a year in clinical rotations.” With former student fellows spreading the word, “recruitment happens naturally,” she says.
Read More »Struggling to find a foothold with NAFLD
March 2020—For pathologists, a first look at nonalcoholic fatty liver disease can be jarring. Purva Gopal, MD, has seen her share of initial biopsies on patients whose “livers are already cirrhotic,” she says. So has Cynthia Guy, MD, professor, Department of Pathology, and chief of the liver and GI surgical pathology section, Duke University Health System. It’s not a good look, obviously, so it’s one she’s doing her best to share with clinical colleagues. At her institution, pathologists have built a strong connection with the hepatologists and gastroenterologists, she says, and NAFLD is part of the regular show-and-tell.
Read More »C. auris: ‘The more we see, the more resistance’
March 2020—While developing into a global health emergency that has killed thousands, the outbreak of the SARS-CoV-2 coronavirus identified in China riveted the public and raised awareness of infectious diseases and their perils.
Read More »For molecular ID testing, micro lab or molecular lab?
March 2020—Where should molecular infectious disease testing be performed? That was the question at the center of a point-counterpoint session at last year’s AMP annual meeting.
Read More »In CML, BCR-ABL monitoring takes on new importance
March 2020—Three decades ago, when Jerald Radich, MD, started doing BCR-ABL testing, the process was highly hands-on. In those days, “we used water baths to do PCR,” said Dr. Radich, an oncologist and director of the molecular oncology lab and member of the Clinical Research Division, Fred Hutchinson Cancer Research Center, in a recent CAP TODAY webinar.
Read More »Addressing the shortcomings of ANA testing by IFA
March 2020—Standardizing indirect immunofluorescence testing for antinuclear antibodies is a critical task for the laboratory community, and it’s more urgent now that new classification criteria make positive ANA a key factor in diagnosing lupus, said Mark H. Wener, MD, in a session at last year’s American Association for Clinical Chemistry annual meeting.
Read More »AMP case report: Advantages of SNP chromosomal microarray over conventional FISH and DNA tests for methylation-specific PCR-positive Prader-Willi syndrome
March 2020—Prader-Willi syndrome (PWS) is a rare genetic multisystem disorder with a reported incidence of approximately one in 15,000. It is characterized by severe infantile hypotonia with feeding problems, global developmental delay and mental deficiency, behavior problems, small hands and feet, hypogonadism and hyperphagia leading to marked obesity in early childhood, and a characteristic face.
Read More »POC panel talks diabetes care, data management, CGM
March 2020—Data management, diabetes care, the demand for continuous glucose monitoring, and device cleansing were up for discussion when CAP TODAY publisher Bob McGonnagle led a point-of-care glucose testing roundtable in January. Joining him were Todd Cullen of Arkray, Corinne Fantz, PhD, DABCC, of Roche, and Susan Fuhrman, MD, of OhioHealth Laboratory Services. Here is what they said.
Study: Combined DNA-RNA testing improves detection of MET mutations
March 2020—DNA and RNA sequencing, when used together, can improve detection of MET exon 14 skipping mutations in lung adenocarcinoma compared with DNA testing alone, according to a study reported last November at the Association for Molecular Pathology meeting. While RNA analysis can play an important complementary role to DNA analysis in detecting the mutation, it can also pick up false-positives if RNA analysis data are not adjusted properly, David Manthei, MD, PhD, a fellow in the University of Michigan Department of Pathology, explained in presenting the data. In the study, 482 cases of non-small cell lung cancer were sequenced using the Oncomine Focus Assay, a Thermo Fisher Scientific next-generation sequencing assay that conducts DNA and RNA analysis in a single workflow.
Read More »Shorts on Standards: Estimating measurement uncertainty
March 2020—The CAP has 30 official liaisons to various organizations who attend scientific meetings or designate others to do so. They report to the Standards Committee, which reports to the Council on Scientific Affairs. We periodically publish bits of what the CAP’s outbound liaisons hear and see in their liaison roles.
Read More »PGx testing wave runs uphill and down
February 2020—Human endeavors are bursting with unintended consequences. Kudzu comes to mind. Smokestacks. Some even point fingers at Smokey Bear. John Greden, MD, offers an example of his own, one with renewed relevance in pharmacogenomics. It’s a subject he’s studied closely, including as principal investigator of the GUIDED trial Researchers looked at whether offering clinicians access to a pharmacogenomics test report would improve treatment for more than 1,100 patients with a major depressive disorder who had already failed to respond to an average of 3.5 antidepressant trials.
Read More »Biomarker screen makes case for MODY genetic testing
February 2020—Cost-effectiveness analysis of health care diagnosis and treatment, unfortunately connoting quotas and spartan budgets, may not have the best reputation among the general public.
Read More »A walk-through on 2 curbside consults
February 2020—In vivo hemolysis accounts for less than two percent of cases of hemolysis, but don’t assume all hemolysis is in vitro. That was the point of one of the patient cases presented at CAP19 in a session on curbside consults in clinical pathology.
Read More »Technological advances enhance liquid biopsy’s clout
February 2020—New microfluidic and nanotechnologies could take liquid biopsy to the next level as a tool to gauge cancer progression and treatment response.
Read More »In memoriam
February 2020—Herbert Derman, MD, CAP president from 1983 to 1985, died Dec. 18, 2019, seeking a remedy to his chronic heart failure. Dr. Derman was CAP vice president from 1981 to 1983 and a member of the Board of Governors from 1971 to 1980. He was the chair and a member of several CAP councils, committees, and commissions. Dr. Derman was named CAP Pathologist of the Year in 1986. He received the ASCP/CAP Pathology Continuing Medical Education award in 1974 and 1978 and the ASCP/CAP Joint Distinguished Service award in 1993.
Read More »Can machine learning algorithms predict lab values?
February 2020—At Massachusetts General Hospital, machine learning is being used in the laboratories to build next-level clinical decision support, and in the latest phase, it’s undergoing trial for use in predicting laboratory results. “I think this is the new paradigm for cost-effective laboratory medicine. This is an important way we’re going to change how we do business,” says Anand Dighe, MD, PhD, who spoke about machine learning techniques for labs during a CAP19 presentation last fall and in a recent interview with CAP TODAY.
Read More »AMP case report: Frameshift and in-frame CALR exon 9 genetic alterations detected in a post-ET myelofibrosis patient before and after stem cell transplantation
February 2020—Myeloproliferative neoplasms (MPNs) are clonal hematopoietic stem cell disorders characterized by the proliferation of one or more of the myeloid lineages. Philadelphia chromosome-negative classical MPNs include polycythemia vera (PV), primary myelofibrosis (PMF), and essential thrombocythemia (ET).
Read More »Using microfluidics to isolate circulating leukemia cells
February 2020—Microfluidic assays are being used to isolate circulating leukemia cells and manage minimal residual disease in some patients with acute myeloid leukemia and B-cell/T-cell acute lymphoblastic leukemia. “There is a lot of popularity in liquid biopsies, but there’s still a lot of work to do,” said Steven A. Soper, PhD, foundation distinguished professor of chemistry, mechanical engineering, and bioengineering at the University of Kansas. Dr. Soper, who also holds a teaching appointment at Ulsan National Institute of Science and Technology in Ulsan, South Korea, was a co-presenter with Sunitha Nagrath, PhD (see story, page 3), at the 2019 AMP annual meeting.
Read More »AP LIS panel: complexity, middleware, reports, AI
February 2020—Middleware, transmitting and consuming reports, and artificial intelligence are just some of what AP LIS roundtable panelists talked about in December with CAP TODAY publisher Bob McGonnagle. Members of the panel were pathologists Monica de Baca, MD, and Jeffrey Prichard, DO, Rick Callahan of NovoPath, David Liberman, MD, of Computer Trust, and Chad Meyers of Sunquest. Here is what they said.
Read More »D-dimer trifecta: clarity on units, values, and use
January 2020—D-dimer has a problem. Several problems, in fact. Many, some might say. Let’s start with the basics regarding D-dimer assays: unit and magnitude. Setting up the equation is easy, and the digits are small: 2 × 4. When applied to D-dimer testing, however, the answer often means far-flung problems for laboratories and clinicians. Parsed out (for fans of James Thurber, this means channeling schoolmarm Miss Groby), D-dimer has two different units of measure. Some assays report fibrinogen equivalent units (FEU), and others measure D-dimer units (DDU).
Read More »No-blot testing charts new course for Lyme Dx
January 2020—“You can eliminate blots altogether!” may sound like a pitch for a cleaning product. But it’s a line from a webpage of Zeus Scientific touting the new algorithm for Lyme disease testing that ditches Western immunoblot in favor of a two-enzyme immunoassay test sequence. The new algorithm, which the CDC recommended last year, is the first approved alternative to the standard algorithm in 25 years.
Read More »Carcinoma of unknown primary case reviewed in tumor board session
January 2020—A molecular oncology tumor board session at CAP19 explored a case of cancer of unknown primary, presented by medical oncologist Alexander Drilon, MD, research director of early drug development at Memorial Sloan Kettering Cancer Center, and Rondell P. Graham, MBBS, head of GI/liver pathology at Mayo Clinic Rochester.
Read More »Study: Subpar reporting of biomarker characteristics
January 2020—Since the Journal of Irreproducible Results arrived on the scene in 1955, founded by a physicist and a virologist, its parodies of scientific research have amused and skewered many in the scientific community. But in the world of clinical research, irreproducibility is a less than whimsical idea.
Read More »At NCI, on the trail of drug-induced liver injury
January 2020—When looking at a liver biopsy, always suspect there might be drug-induced liver injury. “But then try hard to prove there isn’t. It’s always a diagnosis of exclusion and pattern evaluation,” says David E. Kleiner, MD, PhD, reference pathologist for the Drug-Induced Liver Injury Network. In that role, he pieces together clues that point to a drug having injured a liver—or not. Drug-induced liver injury is rare, and few pathologists see it. “The incidence is between one in 100,000 and one in a million, depending on what survey you read,” Dr. Kleiner says.
Read More »New edition of toxicology testing guide now out
January 2020—CAP Publications released this month the second edition of Clinical Toxicology Testing—A Guide for Laboratory Professionals, edited by Barbarajean Magnani, PhD, MD; Tai C. Kwong, PhD; Gwendolyn A. McMillin, PhD; and Alan H.B. Wu, PhD. The first edition was published in 2012. The book has 29 chapters divided into three sections: toxicology testing in the clinical setting, toxicokinetics and methodologies for the toxicology laboratory, and specific analytes (drugs and drug classes). CAP TODAY spoke with Dr. Magnani about the new book. She is director of toxicology and chief of clinical pathology, Department of Pathology and Laboratory Medicine, Tufts Medical Center, and professor of anatomic and clinical pathology, and professor of medicine, Tufts University School of Medicine, Boston. Here is what she told us. (See excerpt below.)
Read More »Cytopathology in focus: Non-small cell lung carcinoma
January 2020—Requests for predictive biomarkers in oncology patients are becoming increasingly common in the cytology laboratory. At the time of rapid on-site evaluation, cytologists are now keenly aware of the need to collect adequate material not just for a diagnosis of malignancy but also for diagnostic and predictive molecular and immunohistochemical testing. This article provides an overview of current practices and some of the recent literature regarding predictive testing for immunotherapy in cytologic preparations in non-small cell lung carcinoma.
Read More »Cytopathology in focus: Self-collected Pap tests in the U.S. market
January 2020—The authors of an article published in the Journal of the American Society of Cytopathology participated in a public hearing at the Food and Drug Administration in January 2018. The hearing advised the FDA about what research would be required to demonstrate the safety and efficacy of a self-collected Papanicolaou test device. In the article, Staats, et al., review the literature on self-collected Pap tests. The authors also provide a review of published studies on self-collected HPV tests. They pose important questions that surround the self-collected Pap test; most remain only partially answered, given the limited evidence examining such tests in the literature.
Read More »Cytopathology in focus: Review of FDA-approved molecular testing platforms for HPV
January 2020—The Food and Drug Administration approved in 2001 the first testing modality for the detection of HPV in gynecological cytological specimens. To date, there are now five FDA-approved testing modalities, and molecular testing for high-risk HPV has become commonplace. Numerous studies have shown that high-risk HPV testing is more sensitive in detecting high-grade squamous intraepithelial lesion/cervical intraepithelial neoplasia grade two and above (HSIL/CIN2+) than cytology alone, but that cytology is more specific.
Read More »No dawdle in switch to high-sensitivity troponin
December 2019—At Mayo Clinic, the latest generation of cardiac troponin assay was an overnight success. Literally. For months, the institution had been preparing to switch to Roche Diagnostics’ Elecsys Troponin T Gen 5 Stat assay, says Bradley S. Karon, MD, PhD, chair of the Division of Clinical Core Laboratory Services, Department of Laboratory Medicine and Pathology. As the time of the rollout neared, an internal medicine colleague who was involved in overseeing the transition asked, “‘What will the burn-in period be?’” recalls Dr. Karon, who is also co-director of Mayo’s stat labs and point-of-care testing programs. Surely clinicians would be able to continue ordering the Gen 4 assay for a time, right? So how long would that last? Dr. Karon’s dramatic-sounding answer? “Zero hours.”
Read More »For gestational diabetes, one step or two?
December 2019—The controversy surrounding the approved methods for screening gestational diabetes mellitus took the form of a debate at this year’s AACC annual meeting, with two speakers defending the one-step or two-step method.
Read More »Labs size up options for unpredictable flu
December 2019—There aren’t good flu seasons; there are just varying degrees of how bad they are. That’s the message the CDC wants people to hear, says Lynnette Brammer, MPH, lead for the CDC’s domestic influenza surveillance team. It’s what laboratories know well and why platforms, panels, and prescribing patterns are top of mind.
Read More »Dodging point-of-care testing potholes in PT, IQCP
December 2019—For point-of-care testing, perform proficiency testing on only one method or instrument unless your testing procedure says all patient samples must be tested on multiple instruments. And if a single IQCP is written for more than one POC testing location, account for all variations.
Read More »Millions at stake in ’21; CAP fights Medicare cuts
December 2019—Medicare reimbursement to pathologists in 2020 is estimated to remain steady, but significant cuts in payments to pathologists and other specialists are expected in 2021 owing to a dramatic shift in how primary care physicians will be paid. The CAP is already fighting the scheduled cuts to pathologists in 2021 as a result of a new plan that reimburses evaluation and management office visit services at a higher rate and lowers payments for non-E/M services billed by specialists.
Read More »LIS roundtable: The conversation continues—consolidation, IT labor force
December 2019—IT as it relates to laboratory consolidation and the labor supply for lab IT were some of what came up when CAP TODAY publisher Bob McGonnagle convened a panel in September to talk about laboratory information systems. Part one of the discussion is in the November issue (with the LIS product guide); part two begins here. On the panel were J. Mark Tuthill, MD, of Henry Ford Health System, Curt Johnson of Orchard Software, Wally Soufi of NovoPath, Michelle Del Guercio of Sunquest Information Systems, Nick Trentadue of Epic, Sepehr Seyedzadeh of Siemens Healthineers, and Tony Barresi of Beckman Coulter.
Read More »Diagnosing GDM in the first trimester
December 2019—“If you thought that diagnosing gestational diabetes at 24 to 28 weeks was unsettled, you haven’t seen anything yet.” That was David B. Sacks, MB, ChB, of the National Institutes of Health, speaking this year in the AACC session on gestational diabetes mellitus (GDM) with his co-presenters who debated the use of the one-step and two-step methods for diagnosing GDM in the second and third trimesters (see story, page 1). His talk: “Let’s Not Wait: Diagnosing GDM in the First Trimester.”
Read More »Panel explores urinalysis solutions, rules, POC testing
December 2019—What do users of urinalysis systems want? According to those in the know, the answer is instruments that are scalable and modular, maximize automation, reduce hands-on time, improve workflow, and more. CAP TODAY publisher Bob McGonnagle convened a panel in October to discuss these topics and other aspects of urinalysis testing. On the panel were Megan Nakashima, MD, of Cleveland Clinic; Michelle Dumonceaux, of Beckman Coulter; Maya Daaboul, of Siemens Healthineers; and Jason Anderson, MPH, MT(ASCP), of Sysmex. What they said follows.
Read More »AMP case report: Identification of a single exon deletion using NGS in a patient with Perlman syndrome
December 2019—We report a case of a 34-week-gestation male infant with prenatal overgrowth born to a 19-year-old G1P0 mother. No significant family history or consanguinity were reported. The infant was delivered emergently by cesarean section due to pregnancy-induced hypertension and polyhydramnios.
Read More »Reflections on biomarkers: so far along, so far to go
December 2019—Presentations at the recent Cancer Biomarkers Conference IV, sponsored by the University of Mississippi Medical Center, prompt reflection on how far we have come since the introduction of Rituxan, the first monoclonal antibody to be approved by the Food and Drug Administration for the treatment of cancer in 1996. Before the launch of Rituxan, physicians were skeptical about the efficacy and safety of monoclonal antibody therapy because of the history of failures.
Read More »Three inside one: biobank, CRO, reference lab
December 2019—Don’t even try to put Boca Biolistics into a box. The Pompano Beach, Fla., company is the rare outside-the-box model of three levels of service under one roof: an expansive biorepository, a contract research organization, and a reference laboratory that earned CAP accreditation in March. Joseph Mauro, president and CEO of Boca Biolistics, has been developing the “new” model for two decades. “The thing that makes us different is that we’re a vertically integrated company encompassing a biobank, CRO, and reference lab. There aren’t many players in this space. LabCorp and Quest have been buying CRO companies, so they are moving into that area, but they are not biobanks. They do not have all of the platforms we have.”
Read More »Mate pair sequencing yields rich new data
November 2019—The LUVOIR telescope proposed this year by NASA, when it is launched into orbit, will outperform the Hubble Telescope 40-fold in ability to detect and visualize deep space objects in detail. But while dazzling in concept, the LUVOIR is still in development. Interestingly, at the genomic level, a similarly impressive advance in detection called mate pair sequencing has already progressed from research to clinical use in diagnosing cancer. With mate pair sequencing, a novel next-generation sequencing technique, Mayo Clinic is advancing the laboratory’s current capabilities for visualizing genetic rearrangements, thus increasing the diagnostic yield of testing for a variety of neoplasms.
Read More »Transgender adult reference intervals taking shape
November 2019—Current sex-specific intervals can be used to interpret hematology results for transgender people using their affirmed gender, say authors of a study published earlier this year.
Read More »Personnel paradox and more: POC pitfalls
November 2019—Point-of-care testing makes up only about 10 percent of all laboratory testing but the aggravation factor and number of people involved far exceeds that, said Deborah A. Perry, MD, medical director of pathology at Methodist Hospital in Omaha, Neb., speaking at CAP19 and calling POC testing “a whole different world.”
Read More »NTRK fusion testing: ups, downs of four methods
November 2019—With two inhibitors approved by the FDA for the treatment of NTRK-fusion-positive solid tumors, the next step is to determine whom to test and how. If the efficacy of the compounds—larotrectinib and entrectinib—were the only thing to consider in implementing a testing algorithm, knowing whom to test would be easy.
Read More »In checklists, new approach for predictive markers
November 2019—In the 2019 edition of the CAP accreditation program checklists, released in September, requirements for predictive marker testing were revised to make them general so they apply to all types of such testing performed by immunohistochemistry and in situ hybridization, wherever possible.
Read More »HemoCell workcell approach brings efficiencies to coag
November 2019—Total laboratory automation solutions, with their integrated, comprehensive approach, have meshed well with the goals of many central labs. But with HemoCell, the first lab automation solution designed for hemostasis testing, Instrumentation Laboratory has shifted gears toward a more specialized solution: a workcell to improve quality and efficiency through process standardization. IL’s HemoCell integrates the company’s ACL Top 750 LAS testing systems, HemoHub Intelligent Data Manager, and HemosIL reagents with Thermo Fisher Scientific’s TCAutomation track. The company’s initial customer base for HemoCell has been in Europe, Asia (particularly China), and South America. Now IL hopes to bring the benefits of HemoCell to more U.S. labs.
Read More »LIS panel talks middleware, wish lists, workflow
November 2019—Middleware, result reporting workflow, consolidation of labs, and IT and laboratory labor were the center of discussion when CAP TODAY publisher Bob McGonnagle convened a panel in September to talk about lab information systems. Part one of the roundtable begins here; part two, on consolidation and labor, will be published in the December issue. On the panel were J. Mark Tuthill, MD, of Henry Ford Health System, Curt Johnson of Orchard Software, Wally Soufi of NovoPath, Michelle Del Guercio of Sunquest Information Systems, Nick Trentadue of Epic, Sepehr Seyedzadeh of Siemens Healthineers, and Tony Barresi of Beckman Coulter.
Read More »Letters
November 2019—We read with interest the case described by Vormittag-Nocito, et al., of metastatic bladder adenocarcinoma with associated molecular pathology findings (September 2019). However, we would like to point out what we believe to be an important update related to the significance of FGFR3 alterations in urothelial carcinoma. When referring to TP53 and FGFR3 alterations in urothelial carcinoma, the authors write that “no targeted therapy or prognostic implications can be made about these mutations at this time.”
Read More »Catching CKD sooner with kidney profile
October 2019—Rarely (as waggish folks like to remind us) is it necessary to reinvent the wheel. Many times it’s better to take existing wheels and stick them on, say, a suitcase. Suddenly, maneuvering through airports becomes 1,000 times easier. Improvement can be that simple, so obvious in retrospect. At least that’s what kidney experts, both inside and outside the laboratory, are hoping as they promote use of the kidney profile lab order to diagnose and monitor chronic kidney disease. Transformation doesn’t always require the thrill of the new. While new markers are always welcome, two stalwart tests—estimated glomerular filtration rate and urine albumin-creatinine ratio—can do plenty.
Read More »For GI cancer, a digital and molecular reset
October 2019—You may not be curling up next to the fire with a cup of hot chocolate to read your copy of the new Digestive System Tumours, part of the World Health Organization Classification of Tumours Series.
Read More »Reporting, other changes in requirements for histocompatibility labs
October 2019—Histocompatibility laboratories that provide services for cellular therapy transplant patients have one more inspection option to consider, thanks to a new edition of the CAP’s histocompatibility checklist released in September.
Read More »For accredited biobanks, a path to CLIA equivalence
October 2019—The requirement revisions in the new edition of the Biorepository Accreditation Program checklist, published last month, are aimed at accommodating a growing overlap between clinical diagnostic activity and biomedical research.
Read More »Faster diagnosis? Chlorinated lipids in sepsis
October 2019—Chlorinated lipids have been shown to be new potential biomarkers for sepsis, and continuing research into their role could lead to faster diagnosis, said David A. Ford, PhD, of Saint Louis University School of Medicine, at this year’s AACC annual meeting. Dr. Ford, a professor of biochemistry and molecular biology, discovered chlorinated lipids in 2002, and at the AACC meeting he shared recent research on the association between chlorinated lipids and lung injury and death in sepsis patients.
Read More »Using predictive analytics to gauge sepsis risk
October 2019—How well can analytics predict the risk for sepsis? T. Scott Isbell, PhD, DABCC, director of clinical chemistry and point-of-care testing, SSM Health, Saint Louis University Hospital, at this year’s AACC annual meeting shared his hospital’s experi-ence with Epic’s sepsis predictive tool. Launched in 2017, the tool uses predictive analytics to produce a sepsis score for ad-mitted patients based on regular scans of key data elements in the electronic health record.
Read More »AMP case report: Use of MYD88 sequencing to confirm diagnosis of PIOL in a case with limited sample availability
October 2019—Primary intraocular lymphoma (PIOL) is a rare but aggressive B-cell malignancy usually considered as a subtype of primary central nervous system lymphoma. The most common form of PIOL is primary vitreoretinal lymphoma. PIOL is also known as the masquerade syndrome because it frequently mimics other ocular conditions such as chronic uveitis, which may be steroid-resistant. Its diagnosis is challenging and requires a high degree of suspicion. Here, we present a case of PIOL, the diagnosis of which was clinched based on the identification of a mutation in the myeloid differentiation factor 88 (MYD88) gene.
Read More »Hematology panel: bridging gaps, staffing, Lab 2.0
October 2019—Automation, the workforce shortage, manual review rates, and Laboratory 2.0 were some of what came up in CAP TODAY’s latest gathering of hematology experts for a roundtable on what’s new, pressing, and in play. CAP TODAY publisher Bob McGonnagle convened a panel in August consisting of Cordelia Sever, MD, of TriCore Reference Laboratories; Olga Pozdnyakova, MD, PhD, of Brigham and Women’s Hospital; Danette Godfrey and Simon Shorter of Sysmex; and Matt Rhyner, PhD, MBA, and Rachel Burnside, PhD, MBA, of Beckman Coulter. What they said follows.
Read More »With real-time data analytics, lab drills down to step it up
October 2019—As payments to laboratories decline and labs look for costs to cut, drawing on Lean and CAP 15189 know-how is the path to stronger productivity, workflow, and quality, “and all of that is eventually going to help,” says Mike Black, MBA, MT(ASCP), DLM, laboratory assistant VP of Avera McKennan Hospital and University Health Center, Sioux Falls, SD, and Avera laboratory service line administrator.
Read More »AP labs diving deeper into automation
September 2019—Talk to a few anatomic pathology laboratory directors about automation, and you may hear references to early network television, when automation’s downsides were mined for comedy. In one interview with CAP TODAY, a lab director drew parallels between potential backups in the automated AP lab and Lucy and Ethel’s travails keeping pace with a chocolate factory conveyor belt. There is strong agreement, to state it in 1960s TV terms, that even for core or centralized AP labs with the necessary volume, the traditional automation options have progressed well beyond the modern Stone Age, but reaching Tomorrowland may require a shift in thinking.
Read More »Shifting trends test labs’ financial mettle
September 2019—There’s nothing new about lab and pathology reimbursement cuts that threaten bottom lines. Over the past three or four decades they’ve become essentially part of the wallpaper in health care.
Read More »New requirements for molecular micro waived testing
September 2019—Four new checklist requirements for waived molecular-based microbiology tests have been added to the CAP point-of-care testing, limited service laboratory, and immunology accreditation program checklists, as part of the 2019 checklist edition released this month.
Read More »Laboratory 2.0: teaming up against opioid use disorder
September 2019—Laboratory pharmacists are a rare breed. When Monique Dodd, PharmD, PhC, MLS(ASCP), spoke at the Executive War College this year, she joked that a third of the laboratory pharmacists in the country might be in the audience.
Read More »Better than glass slides? Digital pathology’s challenge
September 2019—The FDA’s clearance this year of a second digital pathology system for primary diagnosis is likely to lead to lower costs and greater innovation and interoperability, say digital pathology advocates. A multicenter study led the FDA in May to clear Leica Biosystems’ Aperio AT2 DX System for clinical diagnosis in the United States.
Read More »Up-front on PAMA impact, private payer pricing
September 2019—I’m going to talk about our experience over the past couple of years with PAMA, which seeks to produce market pricing, and some of the lessons we’ve learned. We all know how we got here and one of the questions has always been: Did we ever have market pricing? The answer is probably not. When you’re dependent on a 30-plus-year-old fee schedule that never was revised for technology revisions, there wasn’t market pricing to begin with. And at the end of all of what we’re experiencing, we still don’t quite have it.
Read More »Rapid assay detects HIV, syphilis simultaneously
September 2019—The results of a clinical trial of a rapid assay for dual detection of HIV and syphilis were reported at the 2019 HIV Diagnostics Conference in March. If approved by the FDA, where it has been under consideration since 2018, the Chembio Diagnostic Systems assay would be the first point-of-care assay of its kind on the U.S. market.
Read More »AMP case report: NGS of a rare metastatic bladder adenocarcinoma
September 2019—Primary bladder adenocarcinoma is a rare vesicle malignancy accounting for up to two percent of malignant neoplasms of the bladder.1 They occur in males more than females and are classically seen in the fifth or sixth decade of life.2 Histologically they are of enteric, mucinous, or mixed types. Morphologically, the enteric type appears identical to a colonic adenocarcinoma and the mucinous type appears as neoplastic cells floating in pools of extravasated mucin. The mixed type is a mixture of the morphologies of the enteric and mucinous types. Immunohistochemically, adenocarcinomas of the urinary bladder classically express CK20 and CDX2.
Read More »Microbiome swims into our ken
August 2019—Even though he practices in Houston, James Versalovic, MD, PhD, swears he can see the coastline. As he and colleagues at Texas Children’s Hospital delve into research related to the human microbiome, several diagnostic and treatment possibilities are starting to appear tantalizingly close. Comparing their endeavors to ocean explorers of yore, he says, “We’re not quite sure exactly what is in front of us. We can see land—but we’re not quite there.” But unlike Captain Cook and company, Dr. Versalovic and others in the field have access to next-generation sequencing (though Dr. Versalovic jokes, “It’s been around long enough to be called this-generation sequencing”).
Read More »PCT leads the way in antimicrobial stewardship
August 2019—Antibiotic treatment of sepsis patients often has to rely on clinical observation and educated guesswork as clinicians wait for a culture to determine whether the infection is bacterial, viral, or possibly fungal.
Read More »CDC reports on two alternative HIV testing algorithms
August 2019—For HIV testing, a three-step algorithm that differs from the one recommended since 2014 can potentially reduce the number of tests performed and speed up the availability of viral load results, according to a CDC analysis presented at the HIV Diagnostics Conference in March.
Read More »Activated or inactivated? Transfusing the right platelets
August 2019—In the blood bank, all platelets in bags appear to be the same, whether resting or activated, and because they look the same, they may be used as if they’re equal. But they are not.
Read More »Ups and downs of bringing in Beaker AP LIS
August 2019—Having an enterprisewide health care platform can put laboratories in a stronger decision-making position for enterprisewide IT, whereas in most other circumstances, “we are relatively isolated,” said Raj C. Dash, MD, in a talk he gave at this year’s Executive War College. Dr. Dash, vice chair of pathology IT at Duke University Medical Center, shared what he called the blessings and curses of his department’s move in 2014 to a lab information system that’s fully integrated with the electronic medical record. His focus was Beaker’s AP-LIS module.
Read More »AMP case report: Coexisting somatic JAK2 V617F pathogenic variant and likely germline calreticulin exon 9 nonpathogenic variant in a patient with newly diagnosed ET
August 2019–Newly discovered pathogenic variants in BCR-ABL1-negative myeloproliferative neoplasms (i.e. polycythemia vera, essential thrombocythemia, primary myelofibrosis) led to recent revisions of the World Health Organization diagnostic criteria. Initially JAK2 V617F, MPL (MPL W515K/L), and calreticulin (CALR) exon 9 gene pathogenic variants were deemed mutually exclusive in patients with essential thrombocythemia and primary myelofibrosis. However, coexisting somatic variants in both JAK2 V617F and CALR have been reported with variable frequency, ranging from less than one percent and up to 6.8 percent depending on the employed molecular technique.
Read More »In one spot: surgical pathology specimen handling specifics
August 2019—A practical guide that can help labs standardize the handling of a patient’s surgical specimen from harvest to diagnosis is available but too little known, and one of its authors aims to change its hidden treasure status. The 52-page “Practical Guide to Specimen Hand-ling in Surgical Pathology” is on the CAP/NSH Histotechnology Committee page on the CAP website. “Our main objective was to standardize specimen collection handling. Nothing had ever been done like it before,” says Elizabeth Sheppard, MBA, HT(ASCP), past president of the National Society for Histotechnology and head of global market access at Roche Tissue Diagnostics, Tucson, Ariz. She and M. Elizabeth H. Hammond, MD, first chair of the CAP Center Guideline Committee, submitted the topic for an evidence-based guideline; however, it was determined to be better suited as a practical guide for labs to be developed by the CAP/NSH Histotechnology Committee.
Read More » CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management