
Barbara A. Crothers, DO
Edmund S. Cibas, MD
Syed Z. Ali, MD
May 2018—Surgical pathologists take their tumor nomenclature from the WHO Classification of Tumours, but cytopathologists take their terminology from where the consensus groups convened—Bethesda, Paris, Milan, and Yokohama—to formulate terminology recommendations. The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC)1 is now in its second edition. Although the original six diagnostic categories have been retained in their original form, important changes have been incorporated, including notable changes related to the associated clinical management. The terminology is linked to the 2015 American Thyroid Association guidelines for clinical management of thyroid nodules,2 and TBSRTC has become the established terminology for reporting thyroid fine needle aspiration interpretations in the U.S. and elsewhere.
The 2017 TBSRTC monograph, published last fall, includes disease definitions, interpretive criteria, explanatory notes, management strategies, sample reports, and numerous new images that include liquid-based preparations. Notes or comments in a report are not required, but there are several examples to help guide clinical management, especially in the case of findings that might suggest noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP).
The format of the original, and now the 2017, TBSRTC follows the familiar format first established by The Bethesda System for Reporting Cervical Cytology,3 requiring that each report include an adequacy statement, one of a limited number of general diagnostic categories, followed by a diagnostic description of the findings as applicable. TBSRTC requires a general category to facilitate appropriate patient triage by providers who may not be familiar with descriptive diagnostic terminology. As with all efforts to standardize terminology, statistical analysis of outcomes has stronger concordance across practices when uniform, unambiguous terminology is used. This is vitally important in thyroid nodule management as the medical community shifts away from total thyroidectomy for atypical and suspicious nodules to preserve thyroid function.
Current clinical management is directed by evidence-based data that allow for lobectomy for some thyroid cytology interpretations such as suspicious for a follicular neoplasm (SFN) and follicular neoplasm (FN) and lesions suspicious for papillary thyroid carcinoma. Other significant changes are the emerging role for molecular testing in areas of cytologic uncertainty (for example, atypia of undetermined significance [AUS] or follicular lesion of undetermined significance [FLUS] and SFN/FN) and the critical role of ultrasound imaging for the initial and follow-up evaluation of thyroid nodules. Along with fine needle aspiration findings, abnormal ultrasound features may in some cases drive subsequent surgical management or decisions to obtain additional studies.
The six general diagnostic categories remain unchanged from the original TBSRTC: nondiagnostic or unsatisfactory, benign, atypia of undetermined significance or follicular lesion of undetermined significance, follicular neoplasm or suspicious for a follicular neoplasm, suspicious for malignancy, and malignant. The risk of malignancy, or ROM, for each general category has been recalculated from updated published findings of large cohort studies or meta-analyses of data from surgical excisions. In the ideal situation, each laboratory performing both thyroid FNA interpretation and thyroid excision interpretation would calculate its own risk of malignancy based on its accumulated cytologic-histologic correlation data. Meanwhile, the second edition provides an updated risk of malignancy for each category that differs from that in the previous edition, largely as a result of greater consistency in using TBSRTC, robust published data based on large prospective studies, and the recent recategorization of noninvasive follicular thyroid neoplasm with papillary-like nuclear features.4
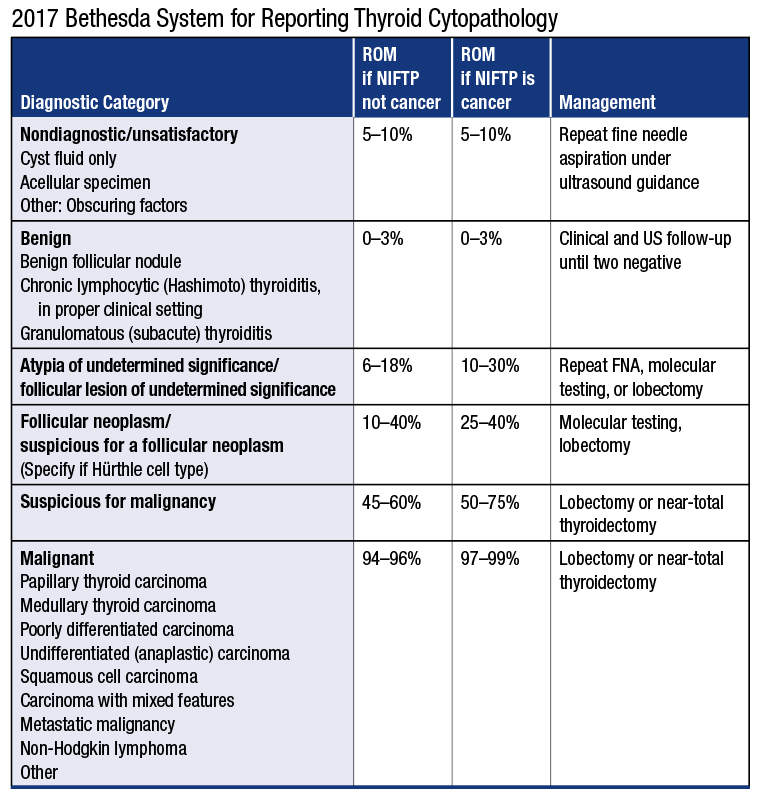
ROM = Risk of malignancy NIFTP = Noninvasive follicular thyroid neoplasm with papillary-like nuclear features
Reprinted by permission from Springer International Publishing. The Bethesda System for Reporting Thyroid Cytopathology. Ali SZ, Cibas E, eds. In: Overview of Diagnostic Terminology and Reporting by Baloch ZW, Cooper DS, Gharib H, Alexander EK. © 2018.
As studies have shown, most NIFTPs, previously called encapsulated follicular variant of papillary carcinoma, are interpreted cytologically as AUS/FLUS, FN/SFN, or suspicious for malignancy. Most behave in a benign fashion and can be treated conservatively, but accurate diagnosis requires a surgical excision. Cytologically, these lesions often show a prominent follicular pattern and have enlarged, slightly irregular nuclei with fewer intranuclear cytoplasmic pseudoinclusions than conventional papillary carcinoma. The 2017 TBSRTC discusses the impact of NIFTP on the risk of malignancy for the various diagnostic categories, and two separate ROM calculations are made, depending on whether or not NIFTP is tallied as a “malignancy.” Excluding NIFTP from the tally of malignancies in the AUS/FLUS, SFN/FN, and suspicious for malignancy categories substantially reduces the risk of malignancy of those categories.
Reaspiration is the suggested follow-up for nondiagnostic (ND) or unsatisfactory (UNS) specimens. Based on ultrasound findings, however, some of these lesions may be sequentially followed (for example, nondiagnostic: cyst fluid only interpretation). A sample with abundant colloid is still considered satisfactory and indicative of a benign lesion even without the minimum numbers of follicular cells, and inflammatory lesions, such as lymphocytic thyroiditis and granulomatous thyroiditis, likewise do not require follicular cells to be considered adequate. Just as in other cytology terminology guidelines, the presence of atypia excludes a nondiagnostic or unsatisfactory interpretation.
The benign category has a very low risk of malignancy and remains the most common interpretation in thyroid cytopathology, encompassing chronic lymphocytic thyroiditis and granulomatous thyroiditis as well as the spectrum of hyperplastic changes. Most of these patients do not require follow-up if they have had two prior benign ultrasound and FNA results.
This edition of TBSRTC clarifies the use of the atypia of undetermined significance or follicular lesion of undetermined significance category. Although pathologists have two terminology choices, the authors of the TBSRTC advise that individual laboratories choose one diagnosis to prevent confusion. These diagnoses are intended to represent the same morphologic spectrum of uncertain cytologic changes, but some laboratories have used AUS for nuclear atypia suggesting papillary carcinoma and FLUS for those without. This approach is strongly discouraged.
To discourage the concomitant use of both AUS and FLUS as separate terms, the authors suggest subcategorizing the atypia in this category as cytologic, architectural, or both cytologic and architectural. Other subclassifications include a sparsely cellular but exclusively Hürthle-cell lesion, atypical lymphoid cells, and atypia, not otherwise specified. AUS/FLUS is a category of last resort and the least reproducible of the six categories. It is recommended that laboratories keep this interpretation below 10 percent of all thyroid cytology reports. A major reason for high AUS/FLUS rates is poor sample quality, often due to scant cellularity, which hinders the assessment of atypia, so this is a metric that can be used in quality improvement programs.
Recommended management options include reaspiration of the lesion if it lacks suspicious ultrasound features. The development of molecular techniques and identification of genetic alterations associated with cancers in the thyroid have led to the introduction of several commercially available tests. They have different strengths and weaknesses and often play a significant role in triaging patients to follow-up or surgery, provided sufficient material is obtained for testing (Zhang M, et al. Arch Pathol Lab Med. 2016; 140[12]:1338–1344).
Similar to AUS/FLUS, suspicious for a follicular neoplasm and follicular neoplasm are synonyms. They encompass the same process and imply the same clinical management. Only one should be used for reporting by any given laboratory. The criteria for SFN/FN are similar to those described previously, with a predominantly microfollicular/crowded architecture, with scant colloid. As a general rule, these lesions should be at least moderately cellular. Notably, the 2017 TBSRTC SFN/FN category now includes lesions with a microfollicular pattern and mild/focal nuclear features of papillary carcinoma, characteristic of NIFTP. Such cases were excluded from SFN/FN in the original TBSRTC. Management is usually a diagnostic lobectomy.
Depending on the degree of atypia, cases with nuclear features that raise the possibility of papillary carcinoma may be classified as either AUS/FLUS or suspicious for malignancy. The Hürthle cell (oncocytic) type of SFN/FN remains a separate subcategory of SFN/FN and distinguished from lymphocytic thyroiditis, where oncocytic nodules are common but frequently accompanied by lymphoid cells. Significantly, many parathyroid adenomas fall into the SFN/FN category and can often be identified accurately with appropriate clinical, laboratory, and radiographic correlation, including parathyroid hormone concentration assays from the intraprocedural needle washout of the aspirate.
Lesions with some but not all the expected cytologic features of malignancy are categorized as suspicious for malignancy and reported as “suspicious for (tumor type).” This category is reserved for cases with cytologic features strongly suggestive of malignancy but which fall quantitatively or qualitatively short of a definitive diagnosis of malignancy. This approach helps to preserve the high positive predictive value of the malignant category.
The malignant category includes papillary carcinoma and its variants, medullary carcinoma, poorly differentiated and undifferentiated carcinoma, squamous cell carcinoma, metastatic tumors, lymphomas, and other rare tumors. These are reported according to the tumor type, mirroring surgical pathology reporting systems. The usual management is total thyroidectomy.
Standardization of cytology terminology for reporting has come a long way since the advent of the Bethesda System for Reporting Cervical Cytology. In addition to the cervix and thyroid, terminology recommendations have been made for the pancreas, urine, breast, and, most recently, salivary gland (See article, page 38). The days of ambiguous, descriptive reporting are over. Cytopathologists have come a long way in clearing the foggy glass of descriptive interpretation and are providing clear and consistent reports for caregivers and patients.
- Ali SZ, Cibas ES, eds. The Bethesda System for Reporting Thyroid Cytopathology, 2nd ed. Cham, Switzerland: Springer; 2017.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.
- Nayar R, Wilbur DC, eds. The Bethesda System for Reporting Cervical Cytology, 3rd ed. Cham, Switzerland: Springer; 2015.
- Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2(8):1023–1029.
Dr. Crothers is the past chair of the CAP Cytopathology Committee and associate professor of pathology at the Uniformed Services University of the Health Sciences, Bethesda, Md. Dr. Cibas is chief of the Division of Cytopathology, Brigham and Women’s Hospital, and professor of pathology, Harvard Medical School, Boston. Dr. Ali is director of the Division of Cytopathology, Johns Hopkins Hospital, and professor of pathology, Johns Hopkins University School of Medicine, Baltimore, Md. Drs. Cibas and Ali are co-editors of The Bethesda System for Reporting Thyroid Cytopathology, and Dr. Crothers is a chapter editor.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
