Karen Titus
February 2015—Like a modern-day Pericles, Tracy George, MD, had much to traverse in her overview of leukocytosis, thrombocytosis, and erythrocytosis during a course on diagnostic hematology at last year’s AACC meeting. Unlike Shakespeare’s Pericles, however, Dr. George navigated the many twists of her topic with the efficiency and near-encyclopedic knowledge of an experienced tour guide.
No magic here, in other words. Just the facts—but compelling ones.
Leukocytosis, the elevation of the white cell count for that patient’s age, by definition requires knowledge of the laboratory’s reference range. Producing a chart of her own lab’s ranges (Fig. 1), she noted that infants and children have reference ranges that are widely different from those of adults. But don’t take her word for it.
“You have to verify this in your own laboratory,” she said, noting that her own lab had recently done this after setting up a new hematology instrument. While validation is a relatively easy exercise for the adult population, “It’s much more challenging for the pediatric reference ranges.”
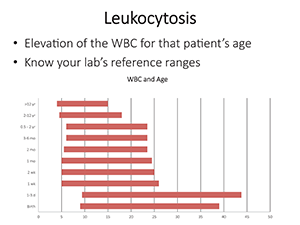
Fig. 1.
Most people use historical results from the literature. Hematology analyzers are, of course, designed to produce accurate, precise blood counts on normal specimens and to alert users to unusual characteristics that could lead to inaccurate measurements or require review of a blood smear. Typical flags, said Dr. George, include blasts, immature granulocytes, nucleated red blood cells, variant or atypical lymphocytes, platelet clumps, giant platelets, or problems with the red cell platelet overlap. Instruments also alert users to abnormalities likely to be associated with spurious results.
Put simply, “Flagged results require review,” said Dr. George, associate professor of pathology and chief of the hematopathology division, University of New Mexico and TriCore Reference Laboratories. “A slide review is appropriate in all patients with an unexplained leukocytosis.” This can be a so-called old-school approach, using a typical manual peripheral blood smear. Dr. George gave the example of a wedge-shaped smear, which, once stained, should be examined for a feathered edge. Laboratories can use newer automated image analysis systems—or even homegrown versions—as well.
Like a Stoic, Dr. George’s philosophy of leukocytosis rests comfortably in the practical. “This is how I think about leukocytosis: You have to confirm your CBC and differential first by examining your blood smear.” The next step in the diagnostic algorithm is to determine whether the case involves increased blasts; a myeloid leukocytosis, such as a granulocytosis or a monocytosis; or a lymphocytosis.
Offering several examples of acute lymphoblastic leukemia, Dr. George noted, in one slide, that at low power one can see an increased white cell count, with many smudge cells in the background. “Very fragile cells,” she said. On higher power, blasts become evident, with very high nuclear-to-cytoplasmic ratios, and with a very thin rim of cytoplasm. Blast size varies. In addition, she noted, anemia and thrombocytopenia accompany patients with acute leukemia, which also will be evident on the slide.
Dr. George would have happily concluded her remarks here, at the four-minute mark (and thus setting some sort of AACC presentation record). “If it was only that simple, right?” she joked, before offering additional examples of lymphoblastic leukemias.
In some cases, the cytoplasm is more abundant. Others, she laughed, “didn’t read the books” on what lymphoblastic leukemias should look like, and feature azurophilic granules. “We recently had a case at our institution [that] had so many granules that we even considered acute promyelocytic leukemia.” In still other cases, there will be L2-type lymphoblasts with abundant cytoplasm, which, in the example she presented, “dares to hug the red cell (Fig. 2). Those are the features we’re supposed to look at for reactive things,” she said, “and it’s mimicking it.”
In contrast, she also showed a case (Fig. 3) of hypogranular acute promyelocytic leukemia with “beautiful” folded nuclei. Those who look hard enough might also find the rare cell with multiple Auer rods to confirm the diagnosis. “Of course, it’s always important to distinguish acute promyelocytic leukemia from acute monocytic leukemia, which has more abundant cytoplasm,” with vacuoles and faint granules.
This was no off-the-cuff remark. “This truly is an emergency at every laboratory.” Such patients have a high risk for DIC and intracranial hemorrhage, so an immediate diagnosis is critical, either by FISH or PCR, looking for the fusion gene PML-retinoic acid receptor alpha, or PML-RARA. “In our own laboratory we start with a rapid cytochemical myeloperoxidase,” Dr. George said.
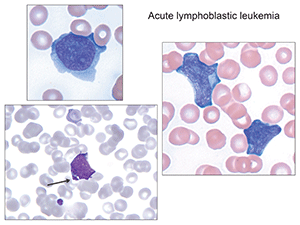
Fig.2. Arrowed image identifies azurophilic granules which can sometimes be seen, so-called granular acute lymphoblastic leukemia. (Images in Figs. 2–6 from Atlas of Peripheral Blood: The Primary Diagnostic Tool. Pereira I, George TI, Arber DA. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2012.)
Labs typically will follow up with flow cytometry. “Most of these cases flow like myeloblasts and have loss or diminished expression of HLA-DR and, typically, CD34. But as we all know, that’s not pathognomonic. So you really have to do the FISH [or] PCR—whichever is faster in your laboratory.” These patients also require bone marrow examination as well as classical cytogenetic karyotyping.
“But you can also see blasts in other things like transient abnormal myelopoiesis associated with Down syndrome,” she continued. Thin, delicately tapered Auer rods are also characteristic in blasts from AML with t(8;21); AMLs can arise from myelodysplastic syndrome as well. These blasts are problematic, Dr. George cautioned, because they’re often smaller than normal myeloblasts. “They can look almost more like lymphocytes.”
If there are increased blasts, a bone marrow exam with flow cytometry, cytogenetics, and molecular genetics studies are in order. “This is just a general rule,” she explained, noting that in rare cases, pathologists are unable to obtain a bone marrow specimen. “We just had an eight-year-old [girl] who was extremely fragile, and we did all the studies on the peripheral blood.”
Lymphocytosis carries its own peculiarities. “If you’ve got a monomorphic lymphocytosis, suspect that you’re dealing with a neoplastic condition,” Dr. George said. On the other hand, in cases involving a pleomorphic lymphocytosis, where the lymphocytes vary in size and shape, “suspect that you’re dealing with a reactive lymphocytosis.”
The classic example of the latter is infectious mononucleosis (“the college student,” as Dr. George put it), with pleomorphic lymphocytosis, some with intensely basophilic cytoplasm. “You can even have more plasmacytic forms” (Fig. 4). It generally occurs at a younger age, with lower white counts— < 50 × 109/L. Good testing options include the Monospot test—the heterophile antibody—which can be done rapidly in most laboratories, or EBV serology. Young children will often be Monospot negative, she said, “so you’ll want to get serology.”
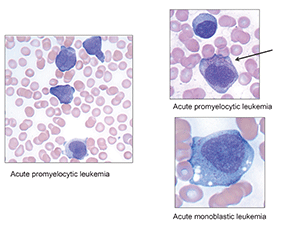
Fig. 3.
What causes reactive lymphocytosis? Among the culprits: viral and bacterial infections, toxoplasmosis, malaria, babesiosis, drug hypersensitivity, autoimmune disease, cytokines, vaccination, smoking, stress (such as trauma, cardiac events, even extreme exercise), endocrine disorders, and even secondary to malignancy.
There are exceptions to those “rules.” Dr. George offered the example of a case of lymphocytosis due to Bordetella pertussis (Fig. 5), the causative agent of “whooping cough,” marked by mature, deeply clefted lymphocytes. Given the patient’s young age, “A quick call to the clinician will confirm that you’re dealing with Bordetella.” It’s also possible to see similar lymphocytes in what’s known as polyclonal B-cell lymphocytosis, which tends to occur in young to middle-aged women who smoke.
In general, a monomorphic lymphocytosis offers a clue to a neoplastic process (Fig. 6). Among these is chronic lymphocytic leukemia, the cells of which have a particular look, to Dr. George’s eye: “I call them the little soccer balls. My colleague calls them snickerdoodles.” Another case, showing prolymphocytes with central nucleoli, was actually a prolymphocytoid transformation of CLL. A case of splenic marginal zone lymphoma showed “tufted” amounts of cytoplasm, what Dr. George called “a bipolar look.” Yet another case showed what looked like blasts. “We thought they were blasts,” she said. “This is why you do flow cytometry.” This case turned out to be a blastic mantle cell lymphoma, “the great mimicker.”
The morphology, in short, provides important clues about the case and how to approach it. “In general, with most of these, we recommend flow cytometry first, and then additional studies based on what we see by flow,” she said.
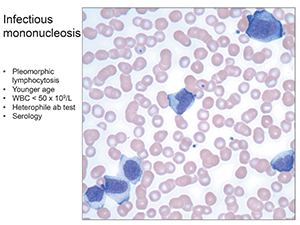
Fig.4. Infectious mononucleosis: Pleomorphic lymphocytosis, younger age, WBC < 50 x 109/L, heterophile ab test, serology.
How often is there peripheral blood involvement in bone marrow lymphoma? Dr. George pointed to a study she and colleagues did (Arber DA, George TI. Am J Surg Pathol. 2005;29:1549–1557) involving nearly 200 samples. Almost 30 percent—57 samples—had involvement of the peripheral blood by morphology and flow cytometry. The study, which excluded CLL, showed a very high rate of involvement by marginal zone lymphoma (eight out of 10 samples, or 80 percent of cases with peripheral blood involvement); mantle cell lymphoma, 48 percent; Burkitt’s lymphoma, 40 percent; diffuse large B-cell lymphoma, 32 percent; and so on. “So it’s not uncommon to see,” she said.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
