Anne Paxton
March 2016—It’s not every day that a monoclonal antibody leads the news. But when former president Jimmy Carter was successfully treated for metastasized melanoma last year with the new drug pembrolizumab (Keytruda), the story made headlines. Carter’s recovery—surprising to many when it was announced in December—may have been helped by traditional radiation and chemotherapy. However, the role played by pembrolizumab spotlighted immunotherapy as an exciting advance in the evolution of cancer treatment.
As its use widens, immunotherapy is ushering in a new leading role for pathology in cancer diagnosis and treatment, and pathologists will need to adapt, says Kenneth J. Bloom, MD, head of oncology and immunotherapy for genomics information technology company Human Longevity in San Diego.
Presenting in a CAP webinar in December, Dr. Bloom detailed how immunotherapy can interrupt the phenomenon of immune checkpoint blockade and the kinds of challenges and dilemmas this process is posing for pathologists. “Instead of focusing only on the tumor and trying to delve deeper and deeper into a small sample from it, now we’re beginning to recognize that the microenvironment might be as essential—and maybe even more essential—for strategies for killing the tumor,” Dr. Bloom says.
People must shift their thinking from targeted therapy—tailored to mutations and the therapies that might best suit them—to immunotherapy, he believes. Because tumor cells have such strong adaptability, they can escape from genomically targeted agents. Immunotherapy addresses this by using the body’s own defenses to allow T cells to expand and kill tumor cells in a variety of malignancies.
Pembrolizumab is one of four major immunotherapy compounds that have either been approved by the Food and Drug Administration or are in phase three trials, and all have a companion diagnostic partner, Dr. Bloom says. Atezolizumab (Genentech) and durvalumab (AstraZeneca) use antibodies from Ventana, while nivolumab (Bristol-Myers Squibb) and pembrolizumab (Merck) use antibodies from Dako. The first two are targeted against PD-L1 (programmed cell death protein 1 pathway) and the second two are targeted against PD-1.
Matching up each drug with a diagnostic involves many more parameters than is typical with targeted therapy, and that can create confusion for pathologists. Testing depends on the indication, Dr. Bloom says. “When a physician asks us to determine PD-L1 status to aid them in determining the efficacy of a particular drug, we need to know not just which drug but what tumor type and the intended use.”
“This is because the basis for the drug approvals might differ; it could be overall survival, progression-free survival, or overall response rate. We also need to know the type of tumor. Is this for a lung cancer and, if so, what type—squamous or nonsquamous? Is this for a melanoma? For bladder cancer?” Then, he adds, the pathologist needs to determine what assay to use, what cells to assess—possibly both tumor cells and immune cells—and what cutoff to use.
“That’s a lot of questions. So when a physician comes up and says, ‘I want to order a PD-L1 test,’ I need to know the answers to all of those questions to best inform them not only which test will be most predictive of response to therapy, but also so that I understand how, as a pathologist, I should interpret the results of that assay.”
With targeted therapy, the pattern has been that the FDA approves the molecule for one indication, generally in a metastatic second- or third-line setting. “Then after a couple of years we see an approval in a first-line setting,” Dr. Bloom says.

Dr. Bloom
With immune drugs, things are happening much faster. Pembrolizumab, for example, an anti-PD-1 drug, was given accelerated approval for unresectable or metastatic melanoma and disease progression following treatment with Yervoy and, if BRAF V600 mutation-positive, a BRAF inhibitor. It has approval, too, for patients with metastatic non-small cell lung cancer whose tumors express PD-L1 as determined by an FDA-approved PD-L1 assay and who have disease progression on or after platinum-containing chemotherapy, as well as for patients with EGFR or ALK genomic tumor aberrations and who have disease progression on FDA-approved therapy for these aberrations prior to receiving pembrolizumab.
Nivolumab has already been approved for unresectable or metastatic melanoma as a single agent (for both BRAF V600 wild-type and mutation-positive tumors) and in combination with Yervoy, as well as for patients who have metastatic NSCLC who have disease progression on or after platinum-containing chemotherapy. More recently it was approved for advanced renal cell carcinoma in patients who have received prior anti-angiogenic therapy.
“These immune drugs appear to be active in so many different tumor types that the rationale for using the drugs, and the approvals for these drugs, will extend over time,” Dr. Bloom says.
In the case of pembrolizumab, data showing an improvement in survival or disease-related symptoms were not initially presented to the FDA. But “that doesn’t mean there wasn’t a survival benefit,” Dr. Bloom says. “The response to the drug looked so beneficial that it got approved before there was data on survival benefit.” Those data were published recently.
When the ongoing trials are completed, the number of indications is likely to expand, but the cutoffs for those indications might change. “For each of the drugs, there is also a unique PD-L1 immunohistochemical assay also approved. Both are produced by Dako. They are pharmDX 28-8 for nivolumab and pharmDX 22C3 for pembrolizumab.”
The trial leading up to the pembrolizumab approval was a phase one study designed to determine the optimal dosage to give, Dr. Bloom says. “They were looking at overall response rate by RECIST criteria and also some secondary endpoints. What was demonstrated was an excellent response rate, either partial response or stable disease, in about 45 percent of patients overall.”Patients who had a tumor proportion score of more than 50 percent—meaning more than 50 percent of the tumor cells were showing some level of expression of PD-L1—showed a significant response rate and duration of response compared with those who had either less expression or no expression of PD-L1, he notes. “It was on this basis that FDA approval was given.”
 The lack of serious side effects was impressive. “If you focus on the grade 3 to 5 adverse events, there were very few that occurred in more than one percent of the patients, and those adverse events—pyrexia, elevation in aspartate aminotransferase, and anemia—are easily treatable. These are incredibly low numbers. The most significant adverse event that you see is fever in about 3.8 percent of patients, but most of the bad side effects are in far less than one percent of the population.” This makes pembrolizumab an extremely well-tolerated drug, especially compared with chemotherapy, he adds.
The lack of serious side effects was impressive. “If you focus on the grade 3 to 5 adverse events, there were very few that occurred in more than one percent of the patients, and those adverse events—pyrexia, elevation in aspartate aminotransferase, and anemia—are easily treatable. These are incredibly low numbers. The most significant adverse event that you see is fever in about 3.8 percent of patients, but most of the bad side effects are in far less than one percent of the population.” This makes pembrolizumab an extremely well-tolerated drug, especially compared with chemotherapy, he adds.
The concept of tumor proportion score, or TPS, was important for the study of PD-L1 pharmDX 22C3 antibody, Dr. Bloom says. “Based on detailed analysis of the clinical trial data, three categories were implemented. ‘No PD-L1 expression’ meant that less than one percent of the tumor cells expressed PD-L1, low expression meant between one and 49 percent of the tumor cells expressed PD-L1, and with high PD-L1 expression, 50 percent or more of the cells showed staining.”
The TPS is the same as the percentage of tumor cells showing staining; that is, number of stained tumor cells divided by the total number of tumor cells. The calculation can get a little tricky because of the difficulty in estimating the number of tumor cells reproducibly and the heterogeneity of PD-L1 expression. (See Fig. 1.)
“If we’ve got a big piece of tumor, it is suggested to break that up into quadrants, calculate the total proportion score in each quadrant, then add them up and divide by four,” Dr. Bloom says. That process works most of the time, if there’s relatively equal distribution of tumor cells in each quadrant. “But if there are wild differences between the number of tumor cells in each quadrant, then you have a more complex calculation.”
Staining patterns can be difficult to read at times, he notes. In the upper left image of Fig. 2 “when it looks like a HER2 stain and we see a strong circumferential thick membranous stain, that’s not a problem. Even in the lower left, where we see basolateral staining, it is relatively easy. It’s where we see some cells with light, granular, or poorly localized staining that it’s difficult to tell.”
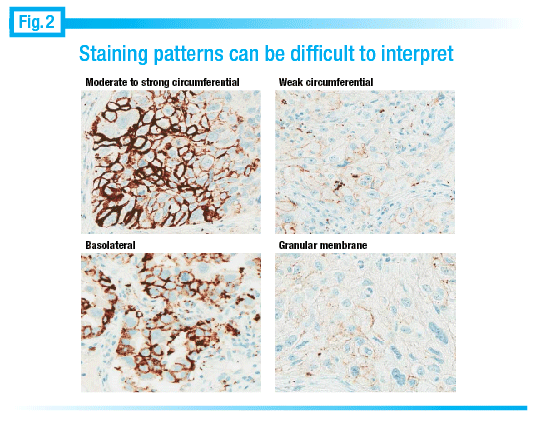 It can be challenging to figure out whether the staining is, in fact, real. “What we’re really looking for is stain that’s localized to the membrane. So cytoplasmic staining doesn’t count; extracellular staining doesn’t count.” In the lower right image of Fig. 2, he notes, “It’s a little bit tough to tell whether that granular staining is really in the cytoplasm or just sitting on the membrane.”
It can be challenging to figure out whether the staining is, in fact, real. “What we’re really looking for is stain that’s localized to the membrane. So cytoplasmic staining doesn’t count; extracellular staining doesn’t count.” In the lower right image of Fig. 2, he notes, “It’s a little bit tough to tell whether that granular staining is really in the cytoplasm or just sitting on the membrane.”
It’s also important to distinguish tumor cells from tumor-associated immune cells because PD-L1 can be expressed on both of them, Dr. Bloom says. “Differentiation can be very challenging in lung cancer where you can have a post-obstructive pneumonia, histiocytes in alveolar spaces lined by tumor, and lymphocytic infiltrates that can strongly express PD-L1.”
“We want to count any membrane staining, at any level of intensity, whether it’s partial or complete, not just the strong ones. Even if there’s very weak staining, as long as we can see it clearly on the membrane, we should call it positive.” Sometimes this can be what he likes to call “hallucinatory staining”—that is, “if you’re not sure whether it’s there or not. It should be clear and distinct membrane staining. Most of the time it’s nice and linear and easy to classify, but sometimes it can be very weak or granular and quite challenging to figure out what percentage is actually on the membrane, versus nonmembranous, which wouldn’t count as positive.”
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
