Kaitlin E. Sundling, MD, PhD
May 2017—Use of cytology specimens for molecular testing can spare patients from repeated or more invasive procedures. A two-part special section in recent issues of Archives of Pathology & Laboratory Medicine highlights a variety of applications of molecular techniques in cytopathology specimens.1–7 The articles in this section cover laboratory workflow issues, considerations for the preparation of cell blocks, application of immunoperoxidase staining and FISH to cytology specimens, and specific applications of molecular testing in thyroid and lung specimens. The main themes are the importance of communication with clinical colleagues to ensure appropriate triage and test ordering, the contribution of preanalytical and analytical factors to the differences between histologic sections of formalin-fixed, paraffin-embedded tissue and cytologic preparations, and the need for future research and development of guidelines for optimal use of cytology specimens for molecular testing. This review will emphasize the spectrum of applications presented in these special section articles.
Integrating molecular diagnostic testing into cytopathology workflow
The articles by Aisner, et al.,1 and Tian, et al.,2 discuss optimizing cytopathology workflows for molecular testing. Aisner and colleagues, of the University of Colorado, emphasize that traditional workflows have evolved with diagnosis being the paramount consideration. Thus, small biopsies and cell blocks may be re-faced multiple times, particularly when immunocytochemistry (ICC) is ordered, sometimes leading to exhaustion of the tissue prior to the opportunity for molecular testing. Two strategies can help: cutting unstained slides up front the first time the block is faced and limiting the use of ICC. To route specimens toward proper processing, communication with clinicians about the purpose of the specimen is critical. Is this a re-biopsy of a known malignancy specifically for molecular testing, or is this specimen primarily intended for diagnosis? Residual tissue may also be requested from the laboratory for clinical trials; thus, conservation of tissue even in routine diagnostic cases may reduce the need for repeat biopsy for clinical trial participation.
The authors suggest embedding small needle core biopsies in multiple blocks for easier triaging of tissue for different testing applications.1 Formalin exposure should be limited; in particular, fixation over the weekend should be avoided. In addition to at least one fine needle aspiration pass, two to four needle cores are requested. FNA specimens are either rinsed directly into formalin or smears are prepared. On needle core biopsy specimens, molecular testing was successful even in relatively low cellularity specimens as long as there were more than 50 cells on initial H&E slides. Overall, implementing dedicated protocols for conserving specimens for molecular testing may increase workload but will improve the success of molecular testing. This has the added potential benefit of avoiding the need for re-biopsy only for the purposes of molecular testing.
Tian and colleagues, of Memorial Sloan Kettering Cancer Center, also suggest concurrent core needle biopsy and FNA, with cell block prepared from FNA needle rinses. In addition to providing high-quality nucleic acid, cytologic specimens have the added advantage of being enriched for tumor cells with relatively little intervening stroma. They prepare a portion of the specimen for molecular testing in a unique way, using components that might normally be discarded. The supernatant of the cell block preparation is centrifuged at high speed. The subsequent pellet is then combined with any residual cell suspension following preparation of the cell block and the ThinPrep slide for DNA extraction and molecular testing. Material from cytologic smears is used only if no other suitable material is available. The dewaxing and washing steps of DNA extraction are identified as key points of DNA loss; thus, to optimize DNA yield, these steps are removed before lysis and proteinase K digestion.
From a bioinformatics perspective, Tian and colleagues describe differential detection of copy number alterations between formalin-fixed and non-formalin-fixed specimens, due to variation in sequencing of GC-rich regions between fixation methods. Thus, use of a similarly prepared control is important for appropriate interpretation of copy number alterations. This issue does not generally affect standard mutational analysis. Overall, the Memorial Sloan Kettering group reports a 90 percent success rate on comprehensive molecular testing of lung cytology specimens with its optimized protocol.
Cell blocks
Cell blocks are an integral part of the workup of malignancy in cytopathology labs. Anjali Saqi, of Columbia University Medical Center, discusses technical aspects of cell block preparation.3 Beginning with specimen collection, a variety of media and preservatives have been used. Saline provides flexibility in later triaging the specimen to cultures, flow cytometry, or cytologic preparations; however, time in saline adversely affects tissue architecture and cytologic detail. Other physiologic salt solutions such as Hank’s buffered saline solution are not addressed. Roswell Park Memorial Institute preservative is useful when subsequent culture and cytogenetic studies are required but may not be readily available. Formalin fixation is validated for IHC and molecular studies but leads to DNA fragmentation. Alcohol fixation leads to comparable or better nucleic acid preservation as compared with formalin, but as will be discussed shortly in the section titled “Lung immunoperoxidase testing,” alcohol-fixed specimens may lead to unreliable results with some IHC stains.
A number of factors may contribute to poor-quality cell blocks.3 Thick, difficult-to-visualize tissue fragments and clots may be left on smears rather than triaged to the cell block. Manual cell block preparation methods may result in dilution of the specimen with excess congealing agent (histogel or plasma/thrombin clot) as well as loss of cellularity owing to incomplete pellet formation. The collodion bag technique seems to yield greater cellularity. The alcohol-based automated method Cellient shows comparable or increased cellular yield as compared with traditional methods, although this method is not easily scalable since one cell block is prepared at a time, taking 45 minutes per block. Although direct smears have been increasingly used for molecular testing, formalin-fixed, paraffin-embedded cell blocks fit more easily into surgical-pathology-oriented workflows. Cell blocks continue to be a focus for currently available EGFR mutation and ALK rearrangement testing guidelines, although updated guidelines are expected.
Thyroid
Zhang and Lin, of Memorial Sloan Kettering Cancer Center, discuss molecular testing of thyroid FNA specimens.4 The current American Thyroid Association guidelines suggest that molecular testing may be performed for the Bethesda System for Reporting Thyroid Cytopathology categories atypia of undetermined significance (AUS/FLUS), follicular neoplasm (FN/SFN), or suspicious for malignancy, depending on clinical utility. The molecular testing panels currently available for thyroid FNA specimens each have differing targets, advantages, and disadvantages.
The Afirma Gene Expression Classifier (GEC) test from Veracyte measures messenger RNA gene expression and has a high negative predictive value and low positive predictive value. ThyGenX from Interspace Diagnostics is a multiplex PCR assay targeting 100 mutations and translocations involving eight genes, including BRAF, RAS, and RET. In contrast to Afirma, ThyGenX has a high PPV and a low NPV. ThyraMIR, also from Interspace Diagnostics, is a microRNA expression test that is meant to be used in conjunction with or subsequent to a negative ThyGenX result. If both ThyGenX and ThyraMIR are negative, the risk of malignancy drops from 32 percent pretest probability to six percent. The independent use of the ThyraMIR test is not addressed. ThyroSeq v2, developed at the University of Pittsburgh and marketed by CBLPath, is a next-generation sequencing method that evaluates more than 1,000 mutations in 14 genes, 42 RNA fusions, and 16 genes for RNA expression. In cases of AUS/FLUS, which have a Bethesda-described rate of malignancy of five to 15 percent, ThyGenX has a high NPV and relatively lower PPV. Thus, ThyroSeq v2 may be better as a rule-out test for malignancy in such cases. ThyroSeq v2 also has a high PPV that may prove sufficient for it to be considered a rule-in test. A fifth option uses the NGS Ion AmpliSeq Cancer Hotspot Panel v2, which has a high NPV but has been studied only on a limited number of cases.
Single-gene BRAF or RAS testing should not be overlooked, as a BRAF V600E mutation in the appropriate context is thought to be diagnostic of papillary thyroid carcinoma, while RAS mutation is more common in follicular neoplasms and noninvasive follicular tumor with papillary-like nuclear features, or NIFTP. American Thyroid Association guidelines do not recommend a specific molecular test to use for thyroid FNA and suggest that additional long-term outcome data are needed in this area. Beyond FNA testing, whole blood evaluation of thyrotropin receptor, which is secreted by thyroid carcinomas but not normal thyroid, may be a helpful adjunct in indeterminate thyroid nodules.
Lung immunoperoxidase testing
Lung is perhaps the clearest-cut example of the clinical utility of molecular testing. Zhou, of MSKCC, and Moreira, of MSKCC and NYU Langone Medical Center, in the first of three articles on lung in the special section, discuss immunoperoxidase (IPOX) testing of cytology specimens.5 Several factors in specimen processing and analysis affect the successful use of molecular alteration-targeted IPOX testing. The authors’ use of IPOX is taken to include both IHC and immunocytochemistry. Preanalytically, alcohol fixation decreases IPOX accuracy, with a decrease in immunogenicity for formalin-optimized antibodies. Forty-three percent of antigens lose immunogenicity in alcohol; however, changes in protocol could improve immunogenicity for most antigens. Placing alcohol-fixed specimens in formalin (i.e. making an FFPE cell block from a liquid-based cytology specimen) restores some immunogenicity. Analytical problems include the subjectivity of interpreting positive staining, and the authors argue for the use of stringent, quantitative cutoffs. Use of quantitative image analysis is not discussed.
Intratumoral heterogeneity is another source of analytical error, as all growth patterns may not be represented on small biopsy or FNA specimens, and distinct growth patterns may represent genetically distinct, prognostically important clones. To this end, the authors recommend increasing the size of biopsies and the range of tumor areas sampled. Specifically addressed tests include EGFR mutation, ALK translocation, ROS1 translocation, BRAF V600E, and PD-L1 IPOX. All of these except PD-L1 have reports in the literature of successful use on cytology specimens. Many abstracts on PD-L1 in cytology specimens have been presented at recent conferences, so perhaps additional publications are in progress.
Generally, molecular alteration-specific antibodies may be considered as screening tests before molecular testing in some scenarios, thus reducing the need for more expensive molecular testing. However, more work is needed to determine the clinical utility of these tests on cytology specimens.
Lung FISH testing
Savic and Bubendorf, of University Hospital Basel in Switzerland, address the applications of fluorescence in situ hybridization to cytology specimens.6 Current molecular testing guidelines recommend use of formalin-fixed, paraffin-embedded tumor material for FISH testing; however, Savic and Bubendorf suggest this is not due to FFPE being the best quality specimen but rather to molecular pathologists having greater exposure to FFPE tissue and less expertise with cytologic specimens. Interpretation of FISH on cytologic whole-cell specimens should be cleaner with more whole nuclei and more intact nucleic acids. FISH has been successful on a variety of cytology specimens, including conventional or liquid-based preparations, Papanicolaou- or Giemsa-stained slides, and specimens after immunocytochemistry. Studies have shown that thresholds for ALK interpretation are similar between FFPE and cytologic specimens. The authors make a side note that ALK IHC may be a reasonable surrogate for ALK translocation testing because native ALK is not expressed in non-neoplastic cells. FISH is capable of detecting other rearrangements, such as ROS1, RET, NTRK1, and NRG1, as well as amplification of MET.
In addition to lung adenocarcinoma, FISH may be useful in delineating mesothelioma from reactive mesothelial cells on cytologic specimens. Interestingly, the authors note that 9p21 deletion and polysomy for chromosome 7 are specific for mesothelioma and can be detected using the UroVysion FISH (Abbott Molecular) assay. The authors suggest that UroVysion may be useful in urine cytology for ruling out high-grade urothelial carcinoma in atypical urothelial cell cases.
Lung biomarker testing perspective from Pulmonary Pathology Society
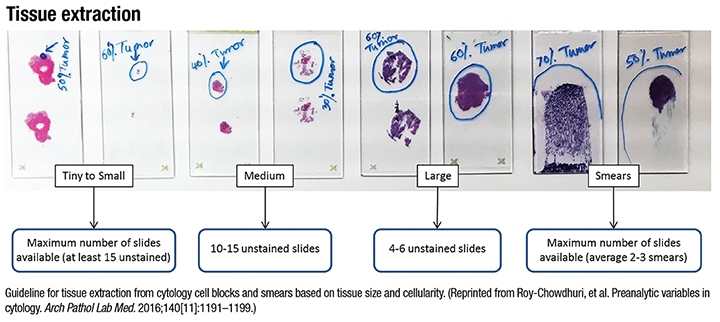
Roy-Chowdhuri and Stewart, of the University of Texas MD Anderson Cancer Center, present a perspective on lung biomarker testing from the Pulmonary Pathology Society.7 The PPS perspective reaffirms the role of cytology specimens in the testing of lung biomarkers. The strongest cases are made for EGFR mutation testing and ALK translocation testing. ROS1 is mentioned in the context of the recent FDA approval of targeted inhibitor crizotinib. Other genes such as BRAF, ERBB2, MET, and RET along with squamous cell carcinoma targets FGFR1, PDGFRA, and PIK3CA are listed as investigational in lung applications. EGFR mutation-specific IHC may be applied to very low cellularity specimens where PCR may be negative, but otherwise it is of limited utility due to a low negative predictive value. ALK FISH testing can be performed on cytology specimens, with appropriately validated protocols. Of note, cell blocks are not specifically addressed in the FDA approval for ALK FISH testing as a companion diagnostic test, but cell blocks are often used if appropriate biopsy material is not available. ALK IHC could be used with cytology specimens as a relatively quick and inexpensive alternative to FISH. Reverse transcription PCR, while traditionally not recommended because it detects only a few well-studied fusion partners, could be used in limited cellularity cytology specimens. A variety of cytology specimen preparations have been used successfully for next-generation sequencing lung mutation panels containing multiple gene targets. Although NGS panels often use relatively high DNA input quantities, non-formalin-fixed cytology specimens generally have higher quality DNA than FFPE tissue and thus may require a lower threshold DNA input to be deemed adequate for testing. (See “Tissue extraction below”)
Summary
The use of cytology specimens for molecular biomarker testing is ever-evolving and represents one of the many ways cytopathologists can provide personalized diagnostic, prognostic, and therapeutic information to patients and clinicians. Careful attention to triaging specimens can maximize the yield of minimally invasive cytology specimens and reduce the need for re-biopsy. Cytology is at the forefront of molecular testing in lung and thyroid, although some guidelines are still fixated on FFPE tissue for molecular testing. Cytopathologists have a key role to play in the future of molecular testing, by guiding their clinical colleagues toward the appropriate use of cytology specimens for molecular testing and by participating in studies to define the performance of these molecular tests on cytology specimens.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
