Anne Paxton
February 2018—To some ears, perhaps, the scientific method connotes a process that is standardized and unimaginative. But inventions like Velcro, vulcanization, and the microwave—all stemming from accidental discoveries—testify to the role of luck and leaps of intuition in formulating and modifying a hypothesis.
When pathologists and scientists at Agilent Technologies, maker of the companion diagnostic PD-L1 IHC 22C3 pharmDx, sought to develop a new scoring methodology, in collaboration with Merck, for gastric cancer patients’ tumor specimens a few years ago, luck and intuition turned out to be handy. As Agilent’s chief pathologist for companion diagnostics, Debra Hanks, MD, puts it, “Skill, science, and a smidgeon of serendipity” helped her team zero in on the best way to evaluate PD-L1 expression in gastric cancer patients.
The result, called the combined positive score (CPS), is part of a companion diagnostics package approved Sept. 22, 2017 by the FDA. The CPS promises to identify more precisely gastric and gastroesophageal junction adenocarcinoma patients who are likely to respond to the drug pembrolizumab (Merck’s Keytruda).
In an Oct. 17 webinar hosted by CAP TODAY with an educational grant from Agilent (available at captodayonline.com), Dr. Hanks describes how pathologists can adopt and perfect their use of the CPS in their own laboratories when analyzing gastric cancer patients’ biopsies, with help from Agilent’s interpretation manual and Web-based training materials for the diagnostic.
The basic mechanism of PD-L1 is well understood: Tumor cells can use the PD-L1 immune cascade to turn off cytotoxic T-cells so the tumor can continue to grow; the antibody pembrolizumab blocks the PD-L1 so the cytotoxic T-cells can continue to stay active and attack the tumor. But “as we learn more and more about immunotherapy, what we’re finding is that different cancer types can express PD-L1 differently,” explains Dr. Hanks, who spoke with CAP TODAY.
Early translational work at Merck Research Laboratories, which studied four tumor types, including gastric cancer, revealed that tumor cell PD-L1 expression was lacking in the majority of those responding to Keytruda. This suggested that the tumor proportion score (TPS), which worked so well in non-small cell lung cancer, would not be a useful biomarker in many other cancers. Interestingly, most of these tumors contained PD-L1-expressing immune cell infiltrates. Scoring immune cell infiltrates was notoriously difficult.
Gastric cancer alone is a disease with a dismal prognosis: five-year survival of 30 percent in the U.S. and five percent worldwide, so the stakes of finding the right scoring methodology for gastric cancer, and other tumor types, were high.
The interpretation criteria for the new CPS methodology are part of the full-solution diagnostic package the FDA approved, explains webinar co-presenter Annika Eklund, PhD, global product manager for Agilent’s companion diagnostics program. “The FDA approved all reagents in the kit, including the 22C3 primary antibody, licensed from Merck; the EnVision Flex visualization system; the control cell lines; the staining procedure on Autostainer Link 48 (software); the instrument Autostainer Link 48 (hardware); and finally the interpretation criteria used by the pathologists in the clinical trial.” Pathologists will find the product insert, Agilent’s interpretation manual, and E-Learning modules to be important sources of information and guidance, Dr. Eklund says. Based on the clinical trial outcomes, the FDA granted accelerated approval in heavily treated PD-L1-positive gastric cancer patients. Through use of the diagnostic PD-L1 IHC 22C3 pharmDx, spurred by the FDA approval, patients with gastric or gastroesophageal junction cancer are already being selected for treatment with pembrolizumab.
“When we collected data for the preliminary clinical trial, called the Keynote-012, Agilent and Merck found that the tumor proportion score . . . did not work well to identify gastric patients,” Dr. Hanks says. The tumor proportion score algorithm identified only two of the 11 gastric patients who responded to Keytruda, so a better method was needed. “We were asked to look at slides from patients who were responders and nonresponders to see if there are any particular staining patterns or expression patterns that would identify responders in gastric cancer.”

Dr. Hanks
The team recorded different staining parameters observed on the slides, breaking down where staining occurred and the amount and intensity, and tried different ways of scoring to develop the new system before arriving at the combined positive score. “Sometimes the process involved trial and error, but it was mainly putting together different formulas and mechanisms by which you could come up with a score, and then seeing if that identified the responders,” Dr. Hanks says. And the scientists and pathologists on the team hit on the key: They discovered that by counting the tumor cells, lymphocytes, and macrophages relative to the viable tumor cells present, they were able to identify nine out of the 11 responders. The combined positive score worked.
To calculate a CPS, the pathologist must score the number of PD-L1-positive cells (tumor cells, lymphocytes, and macrophages), divide that total by the number of viable tumor cells, and multiply by 100. For gastric or gastroesophageal junction adenocarcinoma, a CPS score ≥ 1 identifies responders who are eligible for treatment with pembrolizumab.
To test the value of this experimental algorithm, another clinical study, a phase two clinical trial called Keynote-059, examined the response of the patients identified by CPS to treatment with Keytruda as a third-line monotherapy. To qualify for Keynote-059, the patients had to have shown disease progression on at least two prior treatment regimens and had to have a life expectancy of at least three months, Dr. Hanks says.
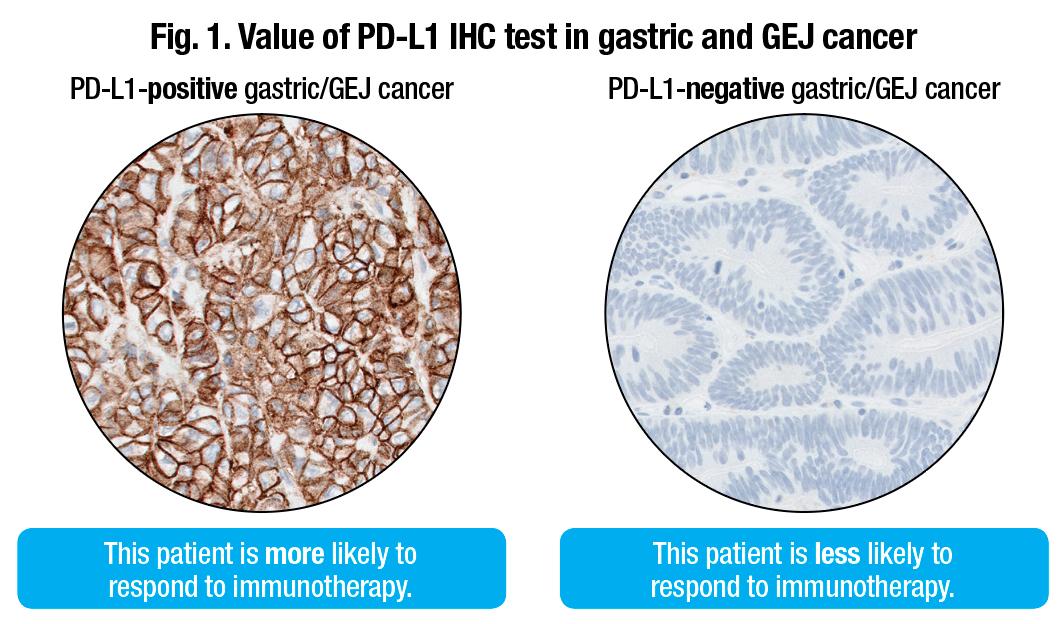 For the 200-plus patients in the Keynote-059 study, about 58 percent had PD-L1 expression (CPS ≥ 1). For the 143 patients who had PD-L1 expression, the overall response rate was 13.3 percent; 1.4 percent had a complete response and 11.9 percent had a partial response (Fig. 1).
For the 200-plus patients in the Keynote-059 study, about 58 percent had PD-L1 expression (CPS ≥ 1). For the 143 patients who had PD-L1 expression, the overall response rate was 13.3 percent; 1.4 percent had a complete response and 11.9 percent had a partial response (Fig. 1).
An important point for pathologists who will be using CPS is the difference between results of testing archival tissue or newly obtained tissue—that is, tissue obtained 42 days before the patient’s first dose of Keytruda. About 49 percent of the cohort studied was positive for PD-L1 if it was archival, but the prevalence rose to 73 percent for newly obtained tissue. Therefore, Dr. Hanks says, “For the drug and our assay label, if you have archival tissue and it tests negative, we recommend that you then obtain and test fresh tissue, to improve the probability of detecting PD-L1.”
Under CAP requirements, pathologists should save blocks and slides for 10 years, she notes, but with the number of biomarkers being identified for companion diagnostics, archival tissue is often an opportunity for oncologists to call the pathologist and order a new test on the patient. “In the case of NSCLC,” Dr. Hanks says, “we have found that blocks that are five years or older could result in a loss of PD-L1 immunoreactivity.”
Agilent’s interpretation manual and four E-Learning modules online will aid pathologists, Dr. Hanks says, in evaluating specimen adequacy and PD-L1 staining results for gastric cancer, calculating the CPS, reporting results, and testing their expertise by scoring a variety of gastric cases, from simple to complex.
Specimen adequacy, naturally, is a key concern for pathologists. “It’s part of our training in pathology to base a diagnosis on the best tissue,” Dr. Hanks says, “to make sure the tissue is adequate and that it’s properly fixed. It doesn’t happen that frequently that we have to deal with preanalytical issues, however, and PD-L1 produces a very robust IHC stain.”
What cells to include in the numerator of the score, and what cells to exclude, are pivotal parts of evaluating the stain, she says, recommending that pathologists keep a copy of the inclusion and exclusion criteria for the algorithm next to their microscope.
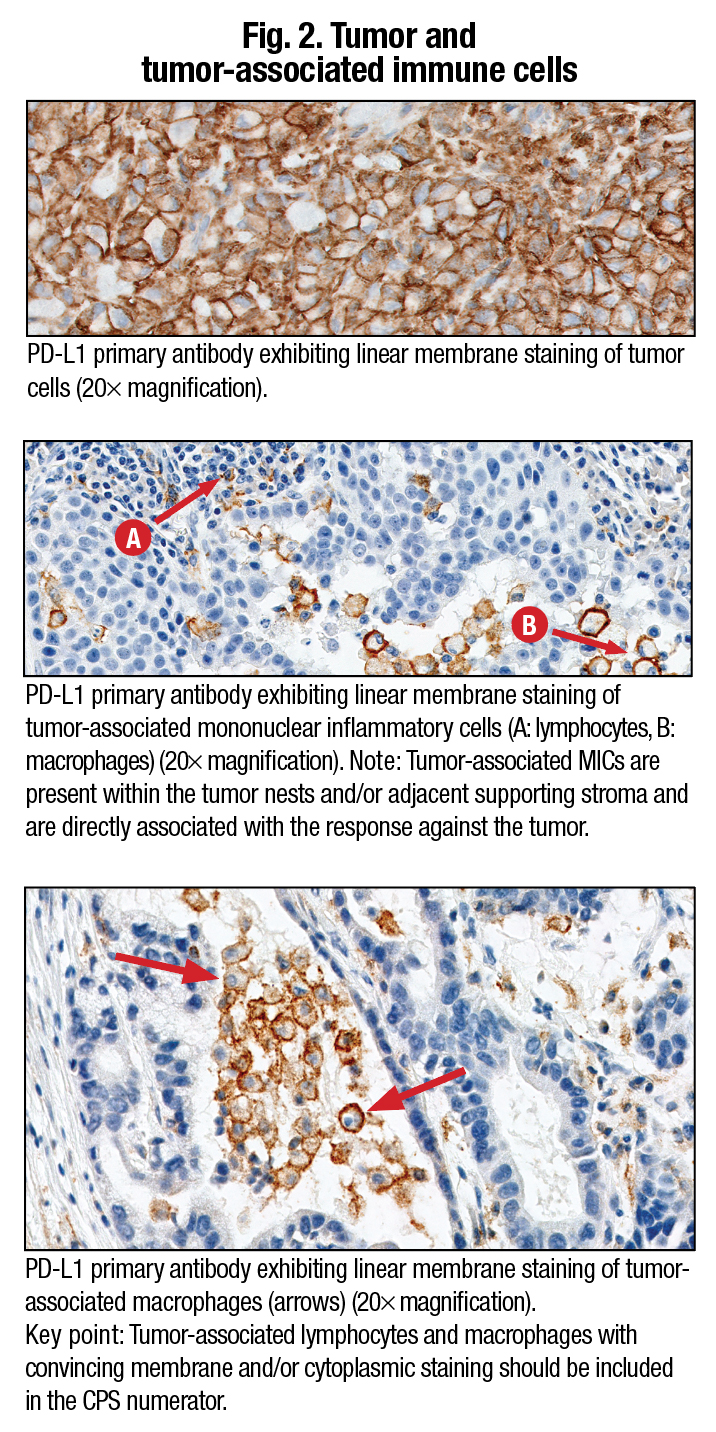 She refers to the images in Fig. 2 to illustrate how the criteria should be employed in calculating the numerator. “The upper image shows positive tumor cells in a beautiful staining pattern that ranges from two-plus to three-plus intensity. These are scored in the exact same way as in our algorithm for non-small cell lung cancer. It’s cell membrane staining. We only score tumor cells in the numerator as positive by membrane staining at any intensity, partial or complete, and it has to be convincing. We do not score tumor cells if they only have cytoplasmic staining; they are considered negative.” A 20× objective is used to decide if weak staining is true membrane staining. “Tumor cells with a small arc of membrane staining are scored as positive,” Dr. Hanks explains.
She refers to the images in Fig. 2 to illustrate how the criteria should be employed in calculating the numerator. “The upper image shows positive tumor cells in a beautiful staining pattern that ranges from two-plus to three-plus intensity. These are scored in the exact same way as in our algorithm for non-small cell lung cancer. It’s cell membrane staining. We only score tumor cells in the numerator as positive by membrane staining at any intensity, partial or complete, and it has to be convincing. We do not score tumor cells if they only have cytoplasmic staining; they are considered negative.” A 20× objective is used to decide if weak staining is true membrane staining. “Tumor cells with a small arc of membrane staining are scored as positive,” Dr. Hanks explains.
The other images in Fig. 2 show a typical immune cell staining pattern for the PD-L1 22C3 kit. “You can count any of the immune cells, whether they be macrophages or lymphocytes, and include them in the numerator. But the key point is that these tumor-associated lymphocytes and macrophages have to have convincing membrane and/or cytoplasmic staining at any intensity to be interpreted as positive.” (Fig. 3).
Sometimes the results can be counterintuitive. “If you only had an H&E, you might review a particular slide and say, ‘This is a poorly differentiated adenocarcinoma of the stomach; this patient has a horrible prognosis.’ But the high amount of PD-L1 staining on a slide of the tumor cells could show the probability that the patient potentially will respond to Keytruda and have an improved prognosis.”
Evaluating the other part of the numerator, the tumor-associated immune cells, can also be difficult. “It’s known from published studies, such as the Blueprint Study”—jointly sponsored by the FDA, American Society of Clinical Oncology, and American Association for Cancer Research to build an evidence base for PD-L1—“that pathologists can have some challenges in scoring immune cells accurately, and so we have worked very diligently with our team of pathologists and scientists to dissect this out and define how to score immune cells. And we’ve included a lot of those caveats, pitfalls, and tips in our literature,” Dr. Hanks says. (Fig. 4).
Deciding what is tumor associated or not is challenging. “We count PD-L1 immune cells associated with the tumor. And when we have a question, we put the tumor in the middle of a 20× field, and any positive immune cells, or what we call mononuclear inflammatory cells [MICs], would be counted. In the same way, if you have a nest of tumor cells and you put it in the middle, any positive membrane and/or cytoplasmic staining at any intensity of the lymphocytes or macrophages are all included in the score.” (Fig. 5).
It’s quite common to see a nest of gastric cancer tumor cells surrounded by PD-L1-positive lymphocytes and macrophages. “We see this staining pattern with PD-L1 quite frequently,” she notes. All of those would count. Lymphoid aggregates are another staining pattern that she refers to as the “mother lode” of positive numerator in the lymphocytes. “These are clusters of lymphocytes that can be found within lots of tumors, and a pathologist can identify their morphology very easily at low power if they are really packed together in a small region.”
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
