January 2024—Thoracic SMARCA4-deficient undifferentiated tumor (TSDUT), formerly known as SMARCA4-deficient thoracic sarcoma and SMARCA4-deficient thoracic sarcomatoid tumor, is a relatively newly defined entity with a distinct clinical history, morphology, immunohistochemical profile, molecular findings, and clinical behavior.
Read More »Bethesda System for Reporting Thyroid Cytopathology
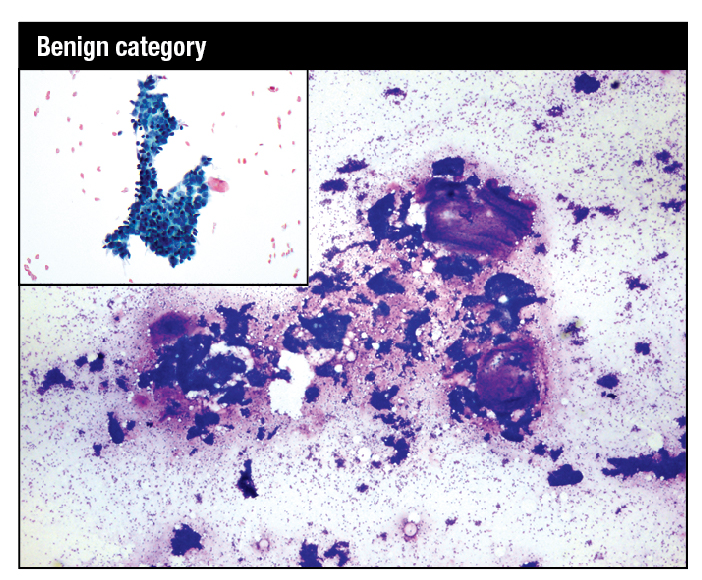
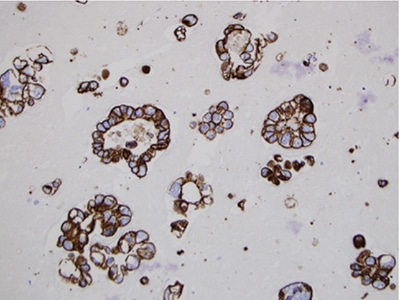
January 2024—The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) is a widely endorsed and globally adapted standardized reporting system of thyroid fine-needle aspiration specimens.
Read More »Reporting urine cytology: how Paris 2.0 differs from 1.0
January 2024—Urinary cytology is widely used to screen for high-grade urothelial carcinoma (HGUC) and to monitor for recurrence. Several reporting systems have been proposed over the past few decades, but The Paris System (TPS) for Reporting Urinary Cytology is the most widely applied worldwide.
Read More »Cytopathology in focus: How to approach cytology of unknown primary
August 2023—We discuss in this article a common problem that all cytopathologists come across frequently in their practice: tumors of unknown primary origin involving body fluids and other sites. Metastatic tumor cells can disseminate and colonize discontinuous secondary body sites.1 Such tumor metastases may be the patient’s initial presenting complaint to a family physician for deep-seated tumor primaries such as ovaries, pancreas, liver, and certain non-obstructive gastrointestinal tumors.
Read More »Cytopathology in focus: Impact of primary HPV testing on cytology lab statistical analysis
August 2023—Cytology gynecologic statistical laboratory data must be analyzed in the context of changing screening modalities and patient populations. This is especially important with the increasing use of primary high-risk HPV screening because diagnostic metrics will be markedly different in that patient population.
Read More »Cytopathology in Focus: Lung cytopathology reporting: WHO system and cases
August 2023—Accurate and timely diagnosis is the cornerstone of effective patient care, particularly in the field of pulmonary pathology. To address the challenges health care professionals face in diagnosing and reporting respiratory conditions, the International Academy of Cytology, together with the International Agency for Research on Cancer, recently developed the World Health Organization Reporting System for Lung Cytopathology
Read More »Inside the WHO Reporting System for Pancreaticobiliary Cytopathology
May 2023—Standardized reporting systems have been developed during the past decade for cytopathology of different organ systems including the pancreaticobiliary system. The Papanicolaou Society of Cytopathology (PSC) in 2014 published the first reporting system for pancreaticobiliary cytology. Studies have demonstrated that implementation of the PSC reporting system has significantly reduced the number of “atypical” interpretations and increased the number of specific diagnoses.
Read More »Adequacy in cytopathology: focus on cytology specimen use in molecular testing
May 2023—In the first article in our series on adequacy in cytology, published in January 2023 (bit.ly/3MDNVzr), we summarized current approaches to defining adequacy for the purpose of primary diagnosis in the majority of specimen types encountered routinely in cytology practice. As we saw, while the essence of adequacy is constant across reporting systems, the technical definitions can vary significantly by specimen type.
Read More »Cytopathology in Focus: Adequacy in cytopathology: an overview with a focus on FNA of lymph nodes and mass lesions
January 2023—The definition of “adequate” per the Merriam-Webster dictionary is “sufficient for a specific need.” In cytopathology, it is defined by the quantity and quality of the cellular material sampled. The final interpretation of a cytopathology report is almost universally preceded by an adequacy statement. While the essence of “adequacy” stays the same, its application varies depending on the specimen type and the site sampled. Furthermore, in the current era of personalized medicine, the definition of adequacy has expanded from “enough cells to make a morphologic diagnosis” to “enough cells to make a diagnosis and perform ancillary studies.”
Read More »Cytopathology in Focus: Serous fluid cytopathology—recent progress and Yale’s experience
January 2023—In recent years, a standardized classification system for the cytology of serous body cavities has been proposed. The system, known as the International System for Reporting Serous Fluid Cytopathology (ISRSFC), published by Ashish Chandra, et al.,1 in 2020, aims to enhance the reproducibility of cytologic diagnoses, thereby facilitating clearer communication with clinicians. The diagnostic categories are as follows: I. Non-Diagnostic; II. Negative for Malignancy; III. Atypia of Undetermined Significance; IV. Suspicious for Malignancy; V. Malignant. Each progressive diagnostic category from I through V carries with it an increasing risk of malignancy. This brief review article aims to highlight the salient points of each diagnostic category and includes discussion of recent publications and our own institutional experience.
Read More »Cytopathology in focus: Protocol for reporting cervicovaginal cytology specimens
August 2022—The protocol for the reporting of cervicovaginal cytology, the first in a series of CAP cytopathology protocols, became available for use in a synoptic format on June 22. This protocol is a collaborative effort, based on input from past and present members of the CAP Cytopathology Committee and prepared in conjunction with the CAP Pathology Electronic Reporting Committee. It was presented via webinar to the CAP House of Delegates on March 31. A two-week open comment period followed; all comments were reviewed and appropriate changes were incorporated into the protocol.
Read More »Cytopathology in focus: ROSE and telecytopathology: a point-of-care test
May 2022—Substantial progress has been made during the past several years in diagnosing and treating various illnesses. Advances in genetic and genomic science; imaging and localization devices; the use of minimally invasive diagnostic sampling procedures; diagnostic, prognostic, and predictive testing; and personalized therapeutic options—all have changed the pattern of the practice of medicine and how patient care is provided.
Read More »Cytopathology in focus: The cytopathology workforce through a DEI lens
May 2022—The ineffectiveness of the U.S. health care system is well documented. The United States consistently allocates more resources for health care compared with other industrialized countries, while not holding the top spots for desired outcomes. A significant percentage of Americans is underinsured or uninsured, and access to quality care is widely asymmetrical among different racial and ethnic groups. Early in the pandemic, COVID-19 highlighted these health inequities in which Blacks, Hispanics, Native Americans, and immigrants were the populations to disproportionately experience disparities related to burden of disease and mortality.
Read More »Cytopathology in focus: Know the accreditation requirements for telecytology
May 2022—The number of minimally invasive fine-needle aspirations requiring rapid on-site evaluation (ROSE) in the cytopathology laboratory has increased over the past decade. Laboratories have seen lower gynecologic volumes and an increase in both nongynecologic fine-needle aspiration biopsy and touch imprint samples. ROSE for patient care has proven value. Sample adequacy allows for a single visit and avoids having to make multiple attempts to provide material sufficient for all required testing, including flow cytometry, microbiology, cell block preparation for immunohistochemical and histochemical staining, and molecular testing.
Read More »Cytopathology in focus: Special stains in the cytology laboratory
January 2022—A consistent virtue of the cytopathology laboratory is that it combines two qualities essential to patient care: It provides an accurate and timely diagnosis. The ability to make a prompt diagnosis is particularly important in immunosuppressed or otherwise vulnerable patient populations for whom a timely diagnosis can result in early treatment initiation and potentially better outcomes.
Read More »Cytopathology in focus: p16 immunostaining in cytology specimens—a diagnostic pitfall
January 2022—Cytopathologists are often the first pathologists to diagnose HPV-related head and neck squamous cell carcinomas (HNSCC). These head and neck cancers can present as superficial masses amenable to fine-needle aspiration, where p16 immunostaining is used as a surrogate marker for HPV in situ hybridization in a subset of squamous cell carcinomas.
Read More »Cytopathology in focus: Statistical reporting—benefits beyond the numbers
January 2022—The CAP has a robust Laboratory Accreditation Program with a commitment to continually improving the programs and providing appropriate resources needed for compliance. As a deemed status organization, validation surveys are performed annually through the Centers for Medicare and Medicaid Services and the feedback obtained provides direction for education.
Read More »Cytopathology in focus: Our appeal to program participants—return glass slides
January 2022—A diverse and copious inventory of Pap and nongynecologic glass slides is the backbone of the CAP glass slide educational programs. Each year tens of thousands of cytopathology slides are packaged and mailed to laboratories enrolled in CAP educational glass slide programs throughout the world. Prior to mailing, numerous cytotechnologists and cytopathologists screen these slides and companion web enhancement images to ensure quality and diagnostic accuracy.
Read More »Cytopathology in focus: Reflections on use of Milan System, edition 1: Areas to be explored for edition 2
August 2021—Salivary gland neoplasms (SGN) are a special group of tumors due to the high variation in histologic subtypes that are further complicated by frequent overlapping morphological features. Fine-needle aspiration is a safe, cost-effective, first-line modality for diagnosing SGNs, an integral part of SGN preoperational workup. In 2018, Faquin and Rossi led the effort to standardize the reporting system of salivary gland lesions. Their final product, Milan System for Reporting Salivary Gland Cytopathology (MSRSGC), has had a huge impact on salivary gland FNA practice in the United States and worldwide.
Read More »Cytopathology in focus: At the center of AI implementation in cytopathology
August 2021—Recent advances in the deep learning area of artificial intelligence offer tantalizing opportunities to improve cytology practice. However, aside from the commercially available options for automated screening in gynecologic cytology, systems with applications in cytology have largely been used in research settings only. The article by authors McAlpine and Michelow reviews the approach to developing and validating artificial intelligence algorithms in cytology, from the generation of appropriate cytology data sets to clinical validation of the model.
Read More »Cytopathology in focus: Inspection pitfalls: Common cytology lab-related deficiencies
May 2021—As COVID-19 restrictions ease, many laboratories are ramping up for biennial CAP inspections. Some of these inspections were delayed due to COVID restrictions and others were performed virtually and now must complete the statutory requirement of an on-site inspection. To add to the mix, the CAP published its 2020 checklist edition earlier than usual because of its impending reapplication with the CMS for deeming authority as an accrediting organization under CLIA. Together, these have made the 2021 inspection process appear unusually daunting. While no laboratory is immune to inspection anxiety, it does help to arm oneself with the knowledge gathered from the collective experiences of peers and colleagues across the country. Knowing what the common inspection pitfalls are can bring us a step closer to the “utopia” of a flawless inspection.
Read More »Primary HPV screen only? Experts warn of risks
January 2021—It’s a watershed moment when an influential standard-setting organization, the American Cancer Society, announces that a test widely used for decades should be supplanted—especially when the test is the linchpin of the most successful cancer screening program in U.S. history.
Read More »Cytopathology in focus: Virtual education in cytology: pandemic silver lining
January 2021—Although the challenges we face due to the COVID-19 pandemic are significant, adapting to our new circumstances can be a driver for positive change. Cytology education had started using virtual learning resources in the past few years with great variation among institutions and countries. The pandemic forced the community to embrace virtual learning in a variety of modalities. These changes may lead to lasting improvements in the quality and accessibility of educational opportunities for cytology trainees, and not just in the United States. In the same way, we are witnessing the exponential growth of digital pathology as applied to surgical pathology. In cytopathology the applications have been limited to telecytopathology in the setting of rapid on-site evaluation (ROSE) for interventional procedures. The difficulties in dealing with multilayered focus have been given as reasons not to pursue whole slide imaging for cytology. As the cytology community has, even if reluctantly, gained experience with remote sign-out, we may see a push for digital imaging for primary diagnosis in cytology.
Read More »Cytopathology in focus: Telecytology for rapid on-site evaluation
January 2021—Rapid on-site evaluation (ROSE) for cytology specimens is performed at many institutions to improve the quality of health care by proper triage of obtained material to increase the diagnostic yield, or to direct appropriate investigation. It also helps to control health care costs by reducing the rate of nondiagnostic specimens, unnecessary passes, and repeat procedures. The number of procedures requiring ROSE is growing due to the increase in the number of platforms used to perform minimally invasive procedures.
Read More »Cytopathology in focus: Direct HPV testing in FNAs from cervical lymph node metastases
May 2020—According to a Centers for Disease Control and Prevention study from 2008 to 2012, there are about 16,000 cases of HPV-positive oropharyngeal squamous cell carcinoma per year in the United States. These carcinomas tend to present with small primary lesion and early nodal metastases as an initial manifestation of the disease. Furthermore, carcinoma of unknown primary presenting as a cystic metastasis in the head and neck has been linked frequently to oropharyngeal squamous cell carcinomas, mainly of palatine tonsils and base of the tongue.
Read More »Cytopathology in focus: Gynecologic cytology PT appeals: where they started,where they stand
May 2020—The CAP implemented proficiency testing for cervical cytology in 2006 as mandated by federal legislation. The performance of participants and granting of appeals on glass slides in the first year of the CAP Pap PT program was reported in detail by Crothers, et al. Once a participant initiates an appeal, the slide in question is pulled from the program for a blinded review by three board-certified anatomic pathologists who are members of the CAP Cytopathology Committee. In the first year, 155 participants failed the PT examination and appealed their testing results on 86 individual slides. After review, appeals were granted for 21 slides, resulting in 45 exam failure reversals. The overall appeal rate was 13/1,000 slides in the program.
Read More »Cytopathology in focus: Can you send us your cytology slides? Labs are reimbursed for slides accepted into programs
May 2020—The CAP relies on the generous submission of slide-based cases from laboratories to maintain the excellent quality of its Cytopathology Educational Programs in Gynecologic Cytopathology (Pap Education), Non-Gynecologic Cytopathology (NGC), Fine Needle Aspiration Cytopathology (FNA), and Proficiency Testing Program in Gynecologic Cytopathology (Pap PT). The CAP Cytopathology Committee, composed of 26 pathologists, two junior members, and two cytotechnologist members, meets quarterly and members submit glass slides to these varied programs.
Read More »Cytopathology in focus: Non-small cell lung carcinoma
January 2020—Requests for predictive biomarkers in oncology patients are becoming increasingly common in the cytology laboratory. At the time of rapid on-site evaluation, cytologists are now keenly aware of the need to collect adequate material not just for a diagnosis of malignancy but also for diagnostic and predictive molecular and immunohistochemical testing. This article provides an overview of current practices and some of the recent literature regarding predictive testing for immunotherapy in cytologic preparations in non-small cell lung carcinoma.
Read More »Cytopathology in focus: Self-collected Pap tests in the U.S. market
January 2020—The authors of an article published in the Journal of the American Society of Cytopathology participated in a public hearing at the Food and Drug Administration in January 2018. The hearing advised the FDA about what research would be required to demonstrate the safety and efficacy of a self-collected Papanicolaou test device. In the article, Staats, et al., review the literature on self-collected Pap tests. The authors also provide a review of published studies on self-collected HPV tests. They pose important questions that surround the self-collected Pap test; most remain only partially answered, given the limited evidence examining such tests in the literature.
Read More »Cytopathology in focus: Review of FDA-approved molecular testing platforms for HPV
January 2020—The Food and Drug Administration approved in 2001 the first testing modality for the detection of HPV in gynecological cytological specimens. To date, there are now five FDA-approved testing modalities, and molecular testing for high-risk HPV has become commonplace. Numerous studies have shown that high-risk HPV testing is more sensitive in detecting high-grade squamous intraepithelial lesion/cervical intraepithelial neoplasia grade two and above (HSIL/CIN2+) than cytology alone, but that cytology is more specific.
Read More »Cytopathology in focus: Lab performance in 2018—a year-end tally
August 2019—The CAP has a long-standing commitment to education in cytopathology, with a number of organized educational offerings in gynecologic and nongynecologic cytopathology. The Interlaboratory Comparison Program in nongynecologic cytopathology (NGC Education) was started in 1997 and the Interlaboratory Comparison Program in fine-needle aspiration glass slide education (FNAG) in 2010. These programs are strictly educational and not graded or used for proficiency testing. Semiannual (FNAG) and quarterly (NGC) mailings include four or five cases. For each case, glass slides generally stained with a Diff-Quik and/or Pap stain are provided.
Read More »Cytopathology in focus: Master’s for all—unifying training in cytotechnology
August 2019—Cytology practice is shifting from fewer gynecologic screening tests to a greater focus on diagnostic testing in nongynecologic cytology including fine-needle aspiration. Roles of cytotechnologists have been changing in the workplace to help laboratories meet new demands and act as pathologist extenders.
Read More »Cytopathology in focus: Updated NSCLC guideline moves molecular cytopathology forward
May 2019—The genomic landscape of non-small cell lung carcinoma is evolving constantly with the discovery of a growing number of molecular alterations and associated targeted therapies that have an impact on patient care. The CAP, International Association for the Study of Lung Cancer, and Association for Molecular Pathology issued a guideline in 2013 to provide a road map for molecular testing to select patients for treatment with targeted tyrosine kinase inhibitors.
Read More »Cytopathology in focus: What pathologist competencies are monitored and how
May 2019—The CAP regularly surveys the practices of the laboratories participating in the CAP Nongynecologic Cytopathology Education Program, or NGC. Members and staff of the CAP Cytopathology Committee developed a supplemental questionnaire eliciting feedback on pathologist competency activities. The Survey was mailed to 2,142 participants in the NGC-B 2018 education program. The pathologist competencies queried were as follows:
Read More »Cytopathology in focus: Exchange of views—HPV screening policies in Australia
May 2019—In the November-December 2018 issue of the Journal of the American Society of Cytopathology is a fascinating analysis of human papillomavirus screening policies in Australia by researchers from New Zealand, a rebuttal by members of an Australian Cervical Cancer Screening Guidelines Working Party, and a thoughtful editorial by cervical cancer screening experts from the United States and England.
Read More »Cytopathology in focus: How can a lab ensure individual competence?
January 2019—It is happening again: CAP members and cytotechnologists are asking about regulatory requirements for re-integrating into cytopathology after a period of practice latency. That is good news because it indicates that they are interested in practicing at a time when the cytopathology community can use skilled professionals. The past decade has seen a shrinking volume of Pap tests and a concomitant decline in the number of practicing cytologists, which has created new job opportunities for those with cytopathology skills.
Read More »Cytopathology in focus: Serous fluid cytology and the international system
January 2019—Just when you thought you were done implementing a new terminology for cytology, another one pops up. Is it possible there are sites that have not yet been standardized? Unbelievably, the most common nongynecologic cytology specimen, body fluids, remains a Wild West for terminology.
Read More »Cytopathology in focus: Next-generation cytotechnology—new cytotechnologist roles
January 2019—The evolution of minimally invasive techniques and new diagnostic modalities have placed new demands on medical laboratories. Cytotechnologists find themselves uniquely poised to take on these new responsibilities, using their morphologic and analytical skills. In this article, I summarize potential roles for cytotechnologists that go beyond screening cytology slides to add value and improve quality in the clinical laboratory.
Read More »Cytopathology in focus: Three special reports capture a field in transition
January 2019—In the September/October 2018 issue of the Journal of the American Society of Cytopathology are three special reports from the American Society of Cytopathology/American Society for Clinical Pathology workgroup on current practices and future perspectives for the field of cytotechnology.
Read More »Cytopathology in focus: ABPath CertLink pilot open to all
January 2019—The American Board of Pathology subspecialty certification examination in cytopathology is given each fall at the ABPath office in Tampa, Fla. Cytopathology has the largest number of examinees of the 11 pathology subspecialty examinations.
Read More »Cytopathology in Focus: Synergy in cytopathology and molecular microbiology
August 2018—In today’s less-is-more world, health care consumers and providers often seek explicit and detailed information from minimally invasive procedures and tiny samples. Over are the days of “malignant cells present” and on to the next case. Cytopathologists and cytotechnologists are embracing and integrating novel techniques and applying new methods to the diagnosis and classification of essentially every imaginable form of neoplasia. The 2018 WHO publications confirm that 29 percent of deaths worldwide (more than 10 million people annually) are attributable to communicable diseases.1,2 This means the purpose of procuring many specimens is not to just rule out malignancy but also to diagnose infectious etiologies.
Read More »Cytopathology in Focus: Why not call everything ASCUS?
August 2018—Below is a question shared on the ASC listserv. My reply to the question follows. A pathologist colleague who practiced previously as an obstetrician/gynecologist is of the opinion that categorizing the level of abnormality we observe on a Pap test is a waste of time. All the clinician needs to know, he says, is whether the test is normal or abnormal. The Pap test is a screening test, he says correctly, and its only relevance is in pointing out who needs a colposcopy and biopsy.
Read More »Cytopathology in Focus: Cytology social media—Facebook and Twitter as networking tools
August 2018—If you are not already using social media professionally, you may not know there is a vibrant and active community of pathologists, including many cytopathologists, on Facebook and Twitter—and getting involved is easy, fun, and educational.
Read More »Cytopathology in Focus: Reporting salivary gland cytopathology—new user-friendly Milan system consists of six diagnostic categories
May 2018—The Milan System for Reporting Salivary Gland Cytopathology was published Jan. 31 and is an important step toward standardizing the reporting of salivary gland fine needle aspiration. A large body of literature has demonstrated that FNA is an effective method for the initial evaluation of salivary gland masses, but until this year there was no uniform, widely accepted reporting system. The complexity of salivary gland cytology poses unique challenges that demand a standardized approach to communication of diagnostic information between pathologists and treating clinicians.
Read More »Cytopathology in Focus: For thyroid cytopathology, the 2017 Bethesda System
May 2018—Surgical pathologists take their tumor nomenclature from the WHO Classification of Tumours, but cytopathologists take their terminology from where the consensus groups convened—Bethesda, Paris, Milan, and Yokohama—to formulate terminology recommendations. The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC)1 is now in its second edition.
Read More »Cytopathology in Focus: Standardized reporting for breast FNAB cytology
January 2018—In countries with developed medical infrastructure, the use of breast fine-needle aspiration biopsy (FNAB) cytology has had its share of challenges over the past 20 years, among them the use of core needle biopsies. In developing countries where the use of FNAB cytology has been increasing rapidly, breast lesions are one of the most common sites sampled by FNAB. In 2016, the International Academy of Cytology Executive Council put together a “Breast Group,” which consists of cytopathologists, surgical pathologists, radiologists, surgeons, and oncologists working in breast care, with the aim of producing a comprehensive and standardized approach to breast FNAB cytology reporting.
Read More »Cytopathology in Focus: A right and a wrong way to use CAP educational kits
January 2018—The CAP Cytopathology Committee constructs educational and interlaboratory comparison kits that are distributed regularly to cytotechnologists, cytopathologists, and pathologists who want continuing education in cytopathology. The purpose of the kits is to make it possible for those who screen and diagnose cytology slides to maintain and update their skills. However, the Cytopathology Committee has been made aware that the kits have been employed for purposes other than education. We address here the potentially detrimental uses to which some laboratories are putting these educational kits and advise laboratories to use them only as they were intended.
Read More »Cytopathology in Focus: Pap proficiency testing: what’s permitted, what’s not
January 2018—Annual proficiency testing is mandated for all cytotechnologists and pathologists who sign out Pap tests. CAP staff frequently receive questions about the PT rules or hear of situations in which there was confusion about the rules, a few of which we will highlight here.
Read More »Cytopathology in Focus: HPV vaccines: the decade in review
January 2018—Diane Harper, MD, MPH, and Leslie DeMars, MD, provide an extensive review of the efficacy of available FDA-approved HPV vaccines in different age groups and describe immunogenicity findings in particular (Gynecol Oncol. 2017;146:196–204). The World Health Organization and the Centers for Disease Control and Prevention recommend a two-dose vaccine for younger children due to high rates of seroconversion and antibody titers in this age group. Girls age 15 and older should continue to get three doses.
Read More »Cytopathology in Focus: Cell Blocks: Getting the most from the least invasive method
August 2017—Adequate and high-quality cell block preparations can be a useful adjunct to cytologic smear preparations and touch imprint cytology. Adequate cell blocks allow for additional studies and can provide a specific diagnosis and information essential for targeted treatment plans. Cell blocks can be prepared from most cytology specimens such as fine needle aspirations, body cavity fluids, washings, brushings, and gynecologic and nongynecologic liquid-based specimens.
Read More »Cytopathology in Focus: Closing cytopathology, cytotechnology practice gaps—three years later
August 2017—The CAP has focused during the past 10 years on facilitating the transformation of pathology practices from the instrumentation age to the information age, with a concentration on personalized medical care and laboratorians as integral members of the health care team. Hand-in-hand with that effort, the CAP Cytopathology Committee has advocated for the expanded use of cytology specimens in molecular diagnostics and the evolution of the cytotechnology workforce to meet the emerging practice gaps as pathologists become engaged in ever more complex diagnostic processes.
Read More »Cytopathology in Focus: More aggressive follow-up for patients with AGC?
May 2017—The most recent edition of the Bethesda System for Reporting Cervical Cytology classifies glandular cell abnormalities into the following broad categories: atypical (specify favored site of origin), atypical (favor neoplastic), endocervical adenocarcinoma in situ (AIS), and adenocarcinoma.1 Generic terminology of “atypical glandular cells (AGC)” may be used if the origin of the cells cannot be determined with certainty. Nevertheless, the Bethesda System encourages pathologists and cytotechnologists to report the favored site of origin (endometrial versus endocervical) whenever possible.
Read More »Cytopathology in Focus: Integrating cytology samples into molecular testing of tumors
May 2017—Use of cytology specimens for molecular testing can spare patients from repeated or more invasive procedures. A two-part special section in recent issues of Archives of Pathology & Laboratory Medicine highlights a variety of applications of molecular techniques in cytopathology specimens. The articles in this section cover laboratory workflow issues, considerations for the preparation of cell blocks, application of immunoperoxidase staining and FISH to cytology specimens, and specific applications of molecular testing in thyroid and lung specimens.
Read More »Cytopathology in Focus: Paris System for urinary cytology: why and where now
May 2017—It is well known that examination of urine dates back to antiquity, but it wasn’t until the 19th century that cancer cells were microscopically documented in urine, by Hermann Lebert in 1845 and Vilem D. Lambl in 1856. Over many decades, countless talented and noteworthy authors have contributed valuable observations and conceptual mechanisms to the study of urinary cytology, but a systematic, universally accepted, internationally recognized system with clear goals was missing.
Read More »Cytopathology In Focus: The significance of NIFTP for thyroid cytology
January 2017—A recent landmark study performed under the auspices of the Endocrine Pathology Society has proposed a new diagnostic entity in the thyroid: noninvasive follicular thyroid neoplasm with papillary-like nuclear features, or NIFTP.
Read More »Cytopathology In Focus: NIFTP’s impact on FNA malignancy risk
January 2017— What’s in a name? As announced in JAMA Oncology in April 2016, tumors previously classified as follicular variant of papillary thyroid carcinoma and found to have no invasion on adequate sampling of the tumor capsule have been given a new name: noninvasive follicular thyroid neoplasm with papillary-like nuclear features, or NIFTP.
Read More »Cytopathology In Focus: CAP meets MOC with à la carte modules online
January 2017—It’s the end of the year, say, and you are just a few self-assessment modules, or SAMs, short of the required 20 in your area of expertise—anatomic pathology. You have to meet the requirements for the American Board of Pathology’s Maintenance of Certification and time-limited cytopathology and AP/CP certificates for each two-year reporting cycle. What to do?
Read More »Cytopathology in Focus: The dysfunctional relationship between labs and their IT
January 2017—Complaints about laboratory information technology services are nearly universal. Rare is the person who is happy with his or her laboratory information system or with the way information services are delivered.
Read More »Cytopathology in Focus—Endoscopic ultrasound-guided FNA and core biopsy: Are we progressing to a best practice?
August 2016—Endoscopic ultrasound (EUS) is a safe and effective procedure for visualizing and screening for lesions within and in the vicinity of the upper gastrointestinal tract, liver, pancreas and peri-pancreatic lymph nodes, and soft tissues. In addition to the detection and imaging of these lesions, endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) allows for concomitant sampling of visualized lesions for tissue diagnosis.
Read More »Cytopathology in Focus: The evolving management of LSIL in Pap tests
August 2016—The Bethesda System for Reporting Cervical/Vaginal Cytologic Diagnoses was developed to establish standardized terminology among pathologists for communicating to clinicians the findings of a Pap test.1 The Bethesda System has also facilitated the examination of the epidemiology and pathogenesis of cervical disease, with a focus on low-grade and high-grade squamous intraepithelial lesions (LSIL and HSIL, respectively) and their relationships to human papillomavirus infection and progression to invasive cervical carcinoma.
Read More »Cytopathology in Focus: Glass slide programs to have latest terms, ancillary clues
August 2016—Why is there more than one correct answer for this glass slide case? I wouldn’t make the diagnosis of mesothelioma without correlating with the clinical history and performing ancillary studies. In our practice, we would call this case a follicular lesion of undetermined significance, and that isn’t an answer on the response sheet.
Read More »Cytopathology in Focus: Paris System: a new paradigm for urinary cytology
May 2016—The Paris System Working Group has proposed and published a standardized reporting system that redefines the primary purpose of urinary cytology: the detection of high-grade urothelial carcinoma (HGUC).1 A program to address standardization of urine cytology reporting was conceived at the 18th International Congress of Cytology in Paris in May 2013 where a number of people of like interest assembled and formed the Paris System Working Group.
Read More »Cytopathology in Focus: Managing adults with thyroid nodules and cancer—2015 guideline highlights
May 2016—In January of this year, the American Thyroid Association published the 2015 update to its guidelines for the management of adults with thyroid nodules and differentiated thyroid cancer.1 Separate guidelines were published for the pediatric population in July 2015.2 Although the guidelines for adult patients were published as a “Special Article” in Thyroid, they run the length of a small book—133 pages in total.
Read More »Cytopathology + More | Assessing needle core biopsy adequacy—survey of practices
May 2016—In the era of personalized medicine1 it is paramount to collect samples that will have sufficient material not only for an accurate diagnosis but also in many cases for prognostication or eligibility for targeted therapy or both. This may involve use of immunohistochemistry, flow cytometry, microbiological culture studies, and molecular studies. Fine needle aspiration and needle core biopsies (NCB) are used routinely for diagnosis of mass lesions from various sites in the body, and both FNA and/or cell blocks and NCB have been used successfully for these purposes.
Read More »Cytopathology + More | Anal cytology: life-saving potential at low cost
January 2016—Anal cancer incidence is on the rise in North America with rates of both invasive and in situ squamous carcinomas of the anus increasing sharply over the past several decades. While women have the highest overall likelihood of developing anal carcinomas, certain male subpopulations (namely men who have sex with men and those who are HIV positive) are at a dramatically increased risk of developing squamous precursors and carcinomas of the anal canal.
Read More »Cytopathology + More | ICD-10: finishing touches or finding the road?
August 2015—To gear up for the change from ICD-9, the Centers for Medicare and Medicaid Services has provided updates and training and has kept ICD-9 changes to a minimum in an effort to build a strong crosswalk to ICD-10. Last year, the U.S. was given one more year to prepare, but that will not be the case this year. In fewer than 75 days, on Oct. 1, the U.S. will convert to ICD-10 coding.
Read More »Cytopathology + More | Primary HPV screening, Pap-HPV cotesting: interim guidance and a retrospective study
August 2015—The Food and Drug Administration in 2001 approved the use of high-risk HPV testing to triage ASCUS Pap test results (reflex testing). Two years later the FDA expanded the indications for hrHPV testing to include its use as an adjunct to cytology in women over age 30 (cotesting). The rationale for age 30 as a cotesting cutoff point was that hrHPV is common in sexually active young women and most infections are transient and clear without medical intervention.
Read More »Cytopathology + More | Telecytopathology’s potential starting to be seen
August 2015—There is a growing body of literature referencing the uses of telecytopathology in clinical care. Telecytopathology is the interpretation of cytopathology material at a distance using digital images. It can be subdivided into three basic applications: rapid on-site evaluation (ROSE), primary specimen diagnosis, and second opinion consultation. Although there is a long history of attempts at implementing telecytopathology for broad clinical use, it still has limited but important applications in patient care.
Read More »Aptima HPV and Aptima genotype assays for triage of borderline squamous (ASCUS) cytology: CLEAR study
May 2015—The characterization of the HPV genome and development of techniques that have the ability to detect nucleic acids in cytologic specimens has had a major impact on patient management. The Hybrid Capture 2 High-Risk HPV DNA Test, or HC2 (Qiagen, Gaithersburg, Md.), which uses probes designed to target the entire HPV genome, was cleared by the FDA in 1996. It was soon realized that determination of clinical sensitivity and specificity was essential to fully characterize assay performance and understand and classify correlation with cervical disease.
Read More »Cytopathology + More | Latest guidelines for pancreatobiliary cytology—a recap
May 2015—Pancreatobiliary malignancy currently accounts for about three percent of all cancer cases and six to seven percent of all cancer deaths, making it the fourth leading cause of death in the U.S. Between 2006 and 2010 the incidence rate of pancreatic cancer increased by 1.3 percent per year and the death rate increased by 0.4 percent per year.1 The incidence of pancreatic cancer has tripled since the 1920s, likely secondary to an aging population, improved disease reporting, and possibly due to increased environmental mutagens2 such as smoking.
Read More »Cytopathology and More | Negative Pap, positive hrHPV: what we know so far
May 2015—Cervical cancer is the second most common cancer in women worldwide, and high-risk human papillomavirus (hrHPV) is considered the principal causative factor in the development of most cervical cancer and its precursor lesions. For this reason, screening algorithms that include testing for hrHPV are part of the new cervical cancer screening guidelines set forth by the American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology.
Read More »Cytopathology and More | Inside the 2014 Bethesda System for Reporting Cervical Cytology
May 2015—The value of standardized terminology for reporting cytology and histopathologyhas been essential in our work and important for patient care. The Bethesda System for Reporting Cervical Cytology, put forward in 1988 thanks to the pioneering work of Diane Solomon, MD, and Robert Kurman, MD,1 saw unprecedented adoption around the world. The Bethesda System, or TBS, led to a number of significant downstream events ...
Read More »Cytopathology and More | Automated screening workload limits are too high
January 2015—A task force of the American Society of Cytopathology in 2009 began the work involved in developing workload recommendations for cytotechnologists who screen image-guided Pap tests. The available data strongly suggested that Pap test screening workloads, as currently approved by the FDA and practiced in some laboratories, are too high and may represent a patient safety risk for the women whose Pap tests are reviewed under those conditions.
Read More »Cytopathology + More | For cytopathologists, MOC exam pass rates and options in 2014
January 2015—Last year was the first year that the American Board of Pathology offered Maintenance of Certification Part III subspecialty examinations. Sixty-four diplomates took a pilot exam in 2013, but it included only anatomic pathology and clinical pathology modules. The secure examination may be taken in years seven to 10 after enrollment in MOC, with no more than 12 years elapsing between examinations. All 2014 examinations were given in Tampa, Fla., but there are plans to offer testing in other locations in the future.
Read More »Cytopathology and More | Touch Imprint/Crush Prep Program better by another name?
January 2015—Do you need a proficiency testing tool for your pathologists and cytotechnologists who perform rapid on-site evaluation? Could you benefit from fine-tuning your cytopathology interpretive skills for the assessment of CT- and ultrasound-guided core biopsies and fine-needle aspirations? Are you looking for a new tool to enhance intraoperative consultation and shorten turnaround time? Why not try the online Touch Imprint/Crush Preparation Program?
Read More »Cytopathology and More | FNA specimens for HPV molecular testing in head and neck SCC
January 2015—Fine-needle aspiration plays a large role at many institutions in the diagnosis of head and neck cancers, and aspiration of enlarged cervical lymph nodes in older individuals is among the more common requests for cytology services. Being that squamous cell carcinoma is by far the most common epithelial malignancy of the head and neck region and that cervical lymphadenopathy in older individuals is one of the more frequent initial manifestations, optimizing these aspirations to direct patient care is a worthy goal.
Read More »Cytopathology and More | Pap proficiency testing—for whom, when, and why
August 2014—It has been almost 10 years since gynecologic cytology proficiency testing, or Pap PT, was implemented in the United States. The CAP is one of three organizations with a Pap proficiency testing program. Pap PT is unique in medicine. In no other situation are licensed physicians or certified technologists required to pass a federally mandated, annual proficiency test before they can practice a skill for which they were trained. Individuals who do not pass Pap PT after two tests cannot practice the interpretation of gynecologic cytopathology until they pass the test.
Read More »Cytopathology and More | ATHENA design, data—and the FDA’s decision
August 2014—The Food and Drug Administration Microbiology Devices Panel of the Medical Devices Advisory Committee held a hearing March 12 on a proposal by Roche Molecular Systems for a new application of human papillomavirus first-line primary cervical cancer screening for women age 25 and older. The 13-member panel unanimously approved the test as safe and effective with benefits to women’s health. The FDA formally approved the additional testing indication on April 24.
Read More »Cytopathology and More | The Pap test under fire
August 2014—The humble Pap test is perhaps one of the most lauded and disdained laboratory tests, lauded because it is the lab test with the best track record of preventing cancer and disdained because the test is labor-intensive, the results are operator dependent, and the regulations are burdensome. Recently the Pap test has come under fire, threatened to be replaced with HPV tests and maligned by patients and physicians for its sometimes unexpected high cost.
Read More »Cytopathology and More | Of confusion, cost, and communication
August 2014—In the days after my “Perspective” piece on the thousand-dollar Pap smear was published,1 I was profoundly moved by the number of physicians from diverse specialties and practice settings who reached out to tell me how important they believe issues of cost and cost transparency are to our ability to practice in the best interest of our patients. Barbara Crothers, DO, of the CAP Cytopathology Committee, was among those who reached out. I learned from Dr. Crothers and her colleagues that pathologists share the sense of frustration and loss of control that I often have as a primary care provider confronted by opaque ordering systems and skyrocketing costs for a simple, potentially life-saving test.
Read More »Cytopathology and More | Cytopathology letter: Ignoring recommendations?
May 2014—Recently I received the 2014 CAP PAPM-A (gynecologic pathology) slide set and was surprised to see case No. 5: a Pap test from an 82-year-old woman with a clinical history of “routine exam.” The cervical cancer screening recommendations from the U.S. Preventive Services Task Force recommend against screening women over the age of 65 who have had adequate prior screening and are not at high risk for cervical cancer, while the American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology guidelines recommend no screening for women over age 65 with evidence of adequate negative prior screening and no history of CIN2+ within the past 20 years.
Read More »Cytopathology and More | Cytopathology at the tipping point
May 2014—A tipping point implies a point of no return, a monumental change in the status quo, a transformation that leads to a new paradigm. Malcolm Gladwell, in The Tipping Point: How Little Things Can Make a Big Difference, popularized the term and defined it as “the moment of critical mass, the threshold, the boiling point.” Tipping points bring both positive and negative consequences; they are a time of change and opportunity. Such is the position that cytopathology finds itself in today.
Read More »Cytopathology and More | FNA cytology: Rapid on-site evaluation—how practice varies
May 2014—Rapid on-site evaluation, or ROSE, is a service that pathologists and cytotechnologists commonly perform to check the cellular content and adequacy of fine-needle aspiration smears and biopsy touch imprints. ROSE can inform the operator of the need to obtain additional samples and, in this cost-conscious age, make it possible to avoid having to repeat the procedure. ROSE allows for preliminary diagnosis so that additional material can be requested for ancillary studies such as flow cytometry, microbiology cultures, or molecular studies.
Read More »Cytopathology and More | Effectiveness of the HPV vaccine in Australia
January 2014—A school-based quadrivalent human papillomavirus vaccine program was introduced in Australia in April 2007 for 12- to 13-year-old girls. This program was also extended to 14- to 17-year-old girls in schools and to 18- to 26-year-old women in the community. The vaccination program has been highly successful, with uptake rates of 86 percent, 82 percent, and 75 percent for doses one, two, and three, respectively. Australia also has an established National Cervical Screening Program. Screening is recommended at age 18, or two years after the onset of sexual activity.
Read More »Cytopathology and More | Evidence emerging for HPV-negative cervical cancer
January 2014—Some studies indicate that nearly all cervical cancers are high-risk human papillomavirus (hrHPV)-related. Recent studies suggested hrHPV testing had a very high sensitivity; therefore, the American Society for Colposcopy and Cervical Pathology recommended Pap cytology and hrHPV co-testing as the preferred screening method in women 30 or older.
Read More »Cytopathology and More | Cytologic-histologic correlation: And the answer is…
August 2013—You have a great gynecologic cytology case, a patient with atypical endometrial cells on Pap test that you believe might represent a low-grade endometrial adenocarcinoma, but it has been four weeks and you have had no feedback about the patient’s outcome. It seems as if there have been a lot of atypical endometrial cells on Pap tests lately. Could it be due to the implementation of a new liquid-based technology for Pap tests in your laboratory? Fortunately, your laboratory performs cytologic-histologic correlation monthly, so you ask the medical director if she has noticed any trends in the rate of atypical glandular cells, and what the corresponding biopsies have shown. To your relief, the patient had a biopsy showing low-grade endometrial carcinoma, and the laboratory statistics have shown only a slight increase in atypical glandular cells since the new technology was implemented. The medical director informs you that she has been recording these data as a special QA project to determine if the increase is due to over-interpretation of reactive glandular cells, because the technology enhances nuclear and cytologic details of glandular cells.
Read More »Cytopathology and More | Managing abnormal screening results: highlights of new guidelines
August 2013—The field of cervical cancer screening saw many developments in 2012. In April last year, the American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology published new guidelines for cervical cancer screening, most notably raising the age at which screening should begin, extending the interval between screening tests, and giving preference to simultaneous Pap and human papillomavirus co-testing in women ages 30 to 65.1 Almost simultaneously, the U.S. Preventive Services Task Force published similar screening guidelines.2 Then, in July, the CAP and ASCCP published the results of a joint project recommending a uniform Lower Anogenital Squamous Terminology (LAST).3 Finally, in September the ASCCP led a consensus conference of 23 participating organizations to update guidelines for managing abnormal cervical cancer screening test results. In April of this year, these updated guidelines were published simultaneously in the Journal of Lower Genital Tract Disease and Obstetrics and Gynecology.4,5
Read More »Cytopathology and more: Interrater agreement of anal cytology
May 2013—Anal-rectal cytology has been used to evaluate HPV-related lesions of the anal canal, particularly in high-risk populations. Because anal cancer is uncommon in the general population, there is no utility in surveillance cytologic assessment on a population-wide scale (as with the Pap test for cervical disease). However, in certain populations, such as men who have sex with men (MSM) and HIV-positive men and women, the risk for anal cancer is higher and approaches the risk of cervical cancer reported in unscreened populations of women. Thus, given that anal cancer shares an HPV-related etiology with cervical cancer and involves a similar squamous mucosal site, anal cytology has been recommended as a method of screening for the prevention of anal cancer through the detection of precancerous lesions (anal intraepithelial neoplasia, AIN). Although the Bethesda terminology, criteria, and guidelines for anal cytology specimens parallel those for cervical cytology, degenerative cellular changes, extensive keratinization, and contaminating fecal material frequently make it more difficult to evaluate these specimens than to evaluate cervical specimens. Because there are limited data on the interobserver agreement of anal cytology (as compared with cervical cytology), Teresa M. Darragh, MD, et al., investigate interrater agreement of anal cytology as well as the relationship between biomarkers and anal cytologic interpretations (Cancer Cytopathol. 2013;121[2]:72–78).
Read More »Cytopathology and more: Know where the deficiencies and dust hide
May 2013—Accreditation inspections are inevitably stressful events. Having a colleague you’ve never met walk through your lab with a checklist looking for how you’ve slipped up reminds me a bit of my mother’s first visit to my apartment when I was a newlywed. I had cleaned everything, including every nook and cranny that weren’t part of my usual routine. I even bought a new shower curtain to ensure no possible hint of mildew. I thought the place looked terrific and that my high level of preparation would surely be sufficient to meet my mom’s white glove standards. Little did I expect that she would peer down the shade of my living room lamp shortly after arriving and remark that I really should dust my light bulbs. It is hard to ever be fully prepared for a June Cleaver mom or to read the mind of a CAP inspector. Fortunately, deficiencies in cytopathology are relatively infrequent and don’t involve eliminating dust on light bulbs. Nonetheless, being aware of the top three checklist items that are most often cited as phase one and two deficiencies can be helpful in your inspection preparation and will assist you in avoiding pitfalls.
Read More »Cytopathology and More | Pathology for the public—a small cascade of realities
May 2013—It seems as though reality TV is taking over the visual airwaves. The shows range from PBS’ traditional and staid “This Old House” to Logo’s “RuPaul’s Drag Race,” in which drag queens are judged by a panel of experts and challenged to “lip-sync for your life.” Who views these shows?
Read More »Cytopathology and More | Endometrial cells in Pap tests—when are they significant?
January 2013—Use of the Papanicolaou test has significantly decreased the incidence of cervical carcinoma, especially cervical squamous cell carcinoma. For endometrial adenocarcinoma, which is the most common malignancy of the gynecologic tract there is no cost-effective screening test. The Bethesda system 2001 recommends reporting normal endometrial cells in women 40 years or older and any atypical endometrial cells under the atypical glandular cells category.
Read More » CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management