Anne Paxton
May 2020—When tick-borne infections are the topic of discussion, talk of Lyme disease, caused by Borrelia burgdorferi, has tended to predominate. But as the prevalence of tick-borne infections in the United States rises steadily and widens geographically, the number of novel pathogens carried by ticks—including non-Lyme bacteria, protozoa, and viruses—has been climbing as well. The growing diversity of tick-borne pathogens is not only changing the conversation, but also posing an array of new challenges for clinical laboratories.
Bobbi Pritt, MD, MSc, DTM&H, chair of the CAP Microbiology Committee, has played a key role since 2011 in discovering and describing two previously unidentified tick-borne disease-causing bacteria, Ehrlichia muris eauclairensis and Borrelia mayonii. These particular species were infecting people not in New England, which once seemed the home base for tick-borne disease, but in the upper Midwest.
“Tick-borne diseases are progressively increasing every year throughout the United States and we’re seeing a number of different tick-borne diseases spreading,” says Dr. Pritt, director of Mayo Clinic’s clinical parasitology lab and co-director, vector-borne diseases laboratory services. Laboratory test volumes confirm the growth trend. One recent study, for example, found there was a 5.3-fold increase in testing for tick-borne infections and a threefold increase in positive results at Massachusetts General Hospital over the 11 years from 2006 to 2017 (Rudolf J, et al. Am J Clin Pathol. 2018;150[5]:415–420).
Increased testing for tick-borne diseases is a contributing factor in such rising numbers, Dr. Pritt and other experts suggest. But the ticks and the infections they carry are definitely on the increase and on the move. They are reaching from the upper Northeast down the east coast, outward from the upper Midwest through the black-legged deer tick (Ixodes scapularis).

Dr. Bobbi Pritt (left) and Dr. Elitza Theel at Mayo Clinic. “Tick-borne diseases are progressively increasing every year throughout the United States and we’re seeing a number of different tick-borne diseases spreading,” Dr. Pritt says.
Lesser known is the Lone Star tick, once confined to the South and Southeast but now found as far north as Wisconsin and parts of New England, Dr. Pritt notes. It does not transmit Lyme disease; it transmits ehrlichiosis and a number of other potentially fatal diseases, including a newly recognized meat allergy. In the western U.S., a tick called the Ixodes pacificus transmits Lyme and some other diseases along the Pacific Coast, while Dermacentor andersoni (Rocky Mountain wood tick) transmits Rocky Mountain spotted fever.
“We need to stop thinking of tick-borne illness as a very localized disease for an endemic area,” says Blake Buchan, PhD, D(ABMM), associate professor at the Medical College of Wisconsin and associate director of clinical microbiology at Wisconsin Diagnostic Laboratories. Several epidemiologic studies have confirmed the increasing range and geographic distribution of ticks carrying these pathogens over the past decade, he says. “It’s very important to recognize that these ticks don’t carry just Borrelia burgdorferi, but also other important pathogens.” More and more labs, he adds, “are recognizing the increased burden of tick-borne illnesses and the increased diversity of organisms that should be in the differential for patients displaying typical symptoms.”
“There are a lot of undiagnosed or misdiagnosed tick-borne infections out there due to the limitations of serologic methods and microscopic examination,” Dr. Buchan continues. “So when you have a positive Lyme serology result, you assume it’s Lyme and may not consider the possibility of acute infection with a different non-Lyme pathogen.” People should be aware of the potential for coinfections with two or more pathogens from one tick species, agrees Elitza S. Theel, PhD, who co-directs with Dr. Pritt the Mayo vector-borne diseases laboratory services.
Laboratories’ traditional use of serologic tests presents a twofold difficulty for purposes of detecting tick-borne diseases, Dr. Buchan says. “First, serologic tests don’t help in diagnosing acute infection because you have a window period before you seroconvert, before that IgM is detectable. With that low sensitivity, the tests don’t help people in the early stages of infection.”
“As far as specificity, many people who are at risk for infection live in relatively endemic areas, so differentiating whether your symptoms are due to a recent infection or your serologic positivity is due to a past resolved infection can be challenging. With a molecular test, if you detect an organism directly, it’s definitive proof of an active infection. You can also pick this up prior to seropositivity, early in the infection during the acute phase when patients may be presenting.”

Dr. Buchan
Dr. Buchan and colleagues, in a recent article, provided an account of instances in which Borrelia burgdorferi was detected with a molecular test (Buchan BW, et al. J Clin Microbiol. 2019;57[11]:e00513-19). “We did that in nine whole blood specimens. None of them were seropositive. These were all acute infections that were completely missed because the patient had not seroconverted yet.” Likewise, he adds, “over 10 percent of the patients seropositive for Lyme were actually positive by the molecular test for a different, non-Lyme pathogen. So these would be misdiagnoses based on seropositivity of an old, resolved infection obscuring or being misleading about what is the current actual cause of your symptoms.”
Historically, Dr. Buchan says, “laboratories such as ours or other small labs in the U.S., maybe after doing a rapid blood smear and Lyme serologic test that’s negative, have sent out specimens for molecular testing at one of the reference labs. And that, of course, delays turnaround time and increases the costs of those results. The expanding commercial availability of reagents that can be used to develop your own laboratory-developed test may help to push this molecular testing into more regional or local high-complexity hospital laboratories. There, those reagents can be used on widely available platforms like the Applied Biosystems 7500 or the DiaSorin Liaison MDX, which are already installed in many labs.”
With the molecular tests, laboratories can say definitively not only which genus it is but also, in some cases, which species, he says. “We’ve seen Ehrlichia, which is historically thought not to be prevalent in Wisconsin because we don’t have the tick vectors. We’ve seen the emergence of a new Ehrlichia species, Ehrlichia muris, which is carried by our Ixodes tick.”
Unfortunately, as of yet there are no FDA-approved molecular tests for any tick-borne diseases, Dr. Pritt says. “Laboratories have to develop their own tests or bring in a test described in the literature and do extensive validation procedures before implementing the test in the laboratory.” Mayo Clinic has individual PCR assays for several non-Lyme diseases, and Dr. Pritt and Dr. Theel have worked with Mayo clinicians to develop an algorithm that labs can follow in using the assays. “There are a lot of different tests out there,” says Dr. Pritt. “You don’t want to just go to the lab catalog and order every test listed.”
Instead, she says, the laboratory needs to ask: “Where in the country are you? What tick-borne diseases are in your area? And then use an algorithmic approach to evaluate for the different diseases.” As the Mayo algorithm shows (this page), some tests should be ordered reflexively in sequence. “Or there may be situations where no testing is indicated.”
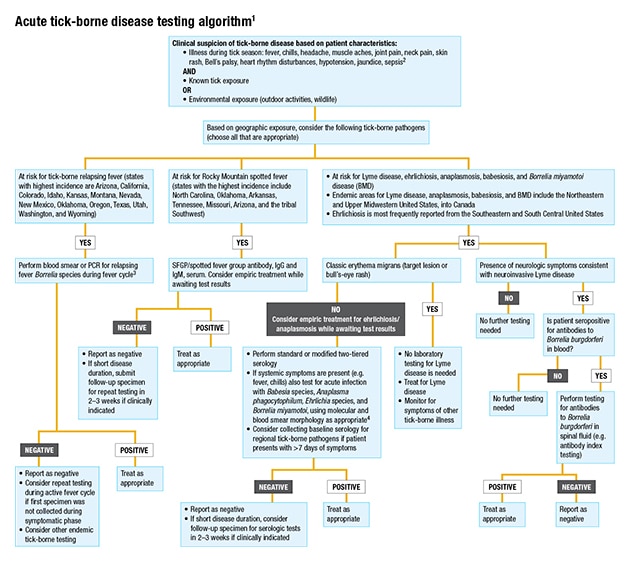
1 Covers testing for the most common tick-borne pathogens in the United States. Not all inclusive.
2 In the presence of severe neurologic symptoms, contact public health authorities for additional testing options (e.g. Powassan/deer tick virus, Heartland virus, Bourbon virus).
3 If infection is thought to have been acquired outside the Americas, order both blood smear and PCR. Not all species of relapsing fever Borrelia may be detectable by a single PCR.
4 Local test availability may vary. PCR testing of blood may be useful for detection of Borrelia mayonii in patients with exposure to ticks in Minnesota or Wisconsin.
The general guidance, Dr. Theel says, when a patient presents with acute symptoms such as fever and myalgias, alongside an appropriate exposure history, is to perform molecular testing for everything except Lyme disease within the first seven days of symptom onset. From eight days after symptom onset, “we then recommend serologic testing be performed, because by that point the immune response has had time to develop and otherwise immunocompetent people will typically have detectable antibodies to the organism.”
It’s hard to make a diagnosis based on a one-time serologic test, Dr. Theel says. “For it to be a significant result, we like to see seroconversion”—negative at time point A and positive when retested at time point B, usually two weeks later. “That is definitive evidence of recent infection. But that’s not helpful in acute settings for patient care. That’s why we recommend molecular testing up front.”
An algorithmic approach like the one developed at Mayo is important for diagnosing tick-borne diseases now, she says. “Pathologists, microbiologists, and laboratories need to work with our patient-facing providers to guide them on what tests they should be ordering,” Dr. Pritt says. “That’s a really important role that laboratory leadership plays.”
The precise cause of the increasing diversity of tick-borne infections is unknown. But several studies suggest that less severe winters in cooler areas of the country such as the Northeast are allowing more larval ticks to survive the winter and helping increase the rate of not only Lyme but also pathogens like Babesia, Anaplasma, and Powassan virus, says Marc R. Couturier, PhD, medical director for infectious disease testing at ARUP Laboratories. He believes the emergence or detection of new species of tick-borne diseases is a side effect of that phenomenon.
“We’ve known about diseases like anaplasmosis and ehrlichiosis for decades, and we’ve seen them in serological testing. But we’re seeing newly emergent pathogens like Heartland virus and Bourbon virus,” he says. “Are those newly existing? Probably not. It’s probably that they were very rare viruses in the tick population, but the number of ticks is becoming larger and larger, so that low-incidence virus is more statistically likely to get exposed to a human.”
The vast majority of testing for tick-borne diseases is conducted by Quest, LabCorp, Mayo Medical Laboratories, and ARUP Laboratories, Dr. Couturier says. However, most other clinical labs are capable of doing the testing with ELISAs or direct fluorescent antibody testing. “These are methods we’ve used for decades. But one of the challenges for smaller labs to do the testing is the seasonality of it.”
“If they’re going to offer the testing, they get slammed during the season and then get almost no orders in the off-season. It can be difficult for a smaller lab to work with that type of ebb and flow, where they basically don’t run a test for months and then all of a sudden are overwhelmed by it,” Dr. Couturier says. “I think that’s why a lot of people continue to send tick-borne disease testing to reference labs. Even in the winter, our volumes don’t die. There’s enough trickling in from enough labs for us to keep the competency and quality assurance up.”
No single test can be used for tick-borne diseases in all situations, he says. “That’s one of the major nuances of tick-borne disease testing. We have an arsenal of weapons available and you need the right test for the right phase of the disease. If the patient has been sick for a while and they are convalescing, then PCR may not be valuable; you may need to do serology on that patient. And vice versa—if that patient is acutely ill, serology would be inappropriate because there’s a good chance they don’t have antibodies yet.”
While molecular testing continues to be laboratory-developed testing—“and not everyone has the bandwidth, capabilities, and patience to do that”—several manufacturers are pursuing FDA clearance for molecular tests, he says. ARUP recently studied the PCR multiplex assay of one such company, ChromaCode, which is hoping to secure FDA clearance and have its assay available on a few different platforms in about a year (Shakir S, et al. J Clin Microbiol. 2020;58[3]:e01655-19). Other companies have had singleplex testing for Babesia on the market for a couple of years, Dr. Couturier adds, but he is not sure how practical it is. “Where Babesia is endemic, you want to know if there is Anaplasma as well. So being able to have multiplexing will be important.”
Multiplexing has the potential, however, to throw too many targets at a patient, not all relevant, he cautions. “So you have a patient in Northern Maine who had an Ixodes tick and is at risk for four things. But the panel that’s on the market includes all the things that the Lone Star tick in the South can vector. If it’s on the panel, they are stuck with it”—along with the risk of false-positives. He hopes the companies can offer a way for laboratories to selectively mask targets they know are not appropriate to their region, or, in other cases where the source of infection is unknown, to decide to test everything.

Dr. Couturier
Patients and sometimes clinicians may assume wrongly that PCR is going to be a better test than serology. “We’ve sung the praises of PCR for so long that it has created this impression that molecular always trumps serology; serology is archaic. But we have plenty of instances with viruses right now where that’s completely untrue. West Nile virus, Zika virus, dengue, and chikungunya are all emerging insect-vectored viruses that are still better diagnosed by serology than by PCR.” It’s not that the PCR is bad. “It’s that the pathogen either hides from our bodies so it’s difficult to sample, or the immune system is clearing the virus quickly. You try to do a PCR and the virus is already gone.”
“For the deer tick virus or Powassan virus, there is good data, especially because it has been increasing lately in prevalence, that PCR testing has a similar narrow window for detection,” Dr. Couturier says. On the other hand, with some of the bacteria and protozoa like Babesia, “when you’re testing the blood, it’s there for a decent amount of time and the nucleic acid can be detected even if the organism is dead. So we find that pathogens like Ehrlichia, Anaplasma, and Babesia, because they live in the blood as their normal cycle, are easy to detect by PCR.” ARUP has had a high rate of positives, upward of 20 percent at times, on those PCR tests, he says.
Lyme bacteria don’t hang around in blood. “They do much better in tissue,” he says. “So if you do a PCR using blood as a specimen, some data out there shows the test may be only 20 percent sensitive. It’s really poor. Whereas if you’re testing a lesion in the tissue where the tick bite was, or the synovial tissue in a joint infected by Lyme, the sensitivity is fantastic.”
The most common target of the multiplex tests he has seen in addition to the Lyme bacterium Borrelia burgdorferi are Anaplasma, Ehrlichia, and Babesia. “These are the core ones that everyone is going after. Some companies are looking at adding the relapsing fever spirochete, a different kind of Borrelia species, or Rickettsia rickettsii or Rocky Mountain spotted fever. We’ve not seen a lot of people pursuing the viruses yet, I think because the availability of positive specimens is scant.”
Comparatively speaking, the cost of serological tests has always been fairly stable and less than molecular testing across the board, Dr. Couturier notes. “While the costs of molecular testing have gone down, they are still a gap higher than serology because of the nature of the reagents. The pricing of PCR has come down over the last 10 years. And the more streamlined it becomes, the more companies can come out with these sample-to-answer tests, where a technician can take a sample, place it into a cartridge, then place the cartridge into an instrument. Now you’re cutting a lot of that labor expense. That’s ideally where we will get to.”
With the wider acquisition of easy-to-use broadly multiplex molecular testing, laboratories will be successful in detecting when specific ticks carry more than one tick-borne pathogen, Dr. Buchan says. “Casting that wider net to recognize those coinfections is important. Continued availability of reagents, be they commercial analyte-specific reagents, kits, or FDA-cleared tests, that can put these tests in the hands of local laboratories, will help increase testing for these non-Lyme pathogens. And that, in turn, should help diagnose a lot of these infections that are currently being misdiagnosed—or being missed altogether.”
Anne Paxton is a writer and attorney in Seattle.