Kristle Haberichter, DO
Domnita Crisan, MD, PhD
Mark A. Micale, PhD
September 2016—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Beaumont Health, Royal Oak, Mich. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
 A 55-year-old male with no significant past medical history presented to an outside institution with a one-month history of a right-sided neck mass. A CT of the neck revealed cervical lymphadenopathy. A needle core biopsy was performed and revealed diffuse intermediate to large cells with anaplastic morphology, including nuclei with horseshoe and wreath shapes (hallmark cells) in a background of small lymphocytes (Fig. 1). Immunohistochemistry confirmed that the neoplastic cells were T cells, with strong and diffuse CD30 expression (Fig. 2). ALK1 staining was performed and was negative. Concurrent flow cytometry studies showed a small population (two percent) of large atypical CD30-positive T cells. Overall, the findings were consistent with an ALK-negative anaplastic large T-cell lymphoma.
A 55-year-old male with no significant past medical history presented to an outside institution with a one-month history of a right-sided neck mass. A CT of the neck revealed cervical lymphadenopathy. A needle core biopsy was performed and revealed diffuse intermediate to large cells with anaplastic morphology, including nuclei with horseshoe and wreath shapes (hallmark cells) in a background of small lymphocytes (Fig. 1). Immunohistochemistry confirmed that the neoplastic cells were T cells, with strong and diffuse CD30 expression (Fig. 2). ALK1 staining was performed and was negative. Concurrent flow cytometry studies showed a small population (two percent) of large atypical CD30-positive T cells. Overall, the findings were consistent with an ALK-negative anaplastic large T-cell lymphoma.
A PET scan for lymphoma staging showed FDG avid adenopathy in the neck, predominantly on the right side, and diffuse bone marrow uptake. A staging bone marrow biopsy was performed at our institution. The bone marrow aspirate showed normoblastic erythroid maturation, granulopoiesis with progressive and full maturation, and increased thrombopoiesis. In addition, there were scattered, pleomorphic large cells with immature nuclei, prominent nucleoli, irregular nuclear contours, and agranular basophilic cytoplasm. Occasional binucleated cells and mitotic figures were identified. These neoplastic cells represented five percent of all nucleated elements.
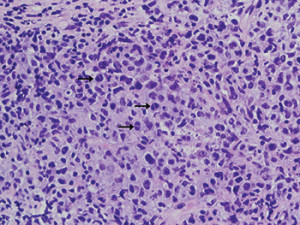
Fig. 1. Right neck mass needle core biopsy showing intermediate to large cells with anaplastic morphology, including hallmark cells (black arrows). H&E staining at 500× magnification.
The bone marrow core biopsy had a highly variable cellularity, ranging from markedly hypocellular areas (less than five percent) to areas of normal to hypercellularity (50–80 percent). There was an extensive abnormal infiltrate composed of the large neoplastic cells forming very large clusters (Fig. 3). Increased mitotic activity was observed in these areas. The neoplastic cells replaced the normal trilineage hematopoiesis and composed 40 percent of the marrow cellularity. Immunohistochemical staining confirmed anaplastic large T-cell lymphoma, CD30-positive and ALK-negative.
Cytogenetic studies were performed on the bone marrow biopsy and demonstrated one normal and one abnormal cell line with a complex karyotype (Fig. 4). Four of 20 cells presented an abnormal male karyotype with 46 chromosomes that included a translocation involving the long arm of chromosomes 1 and 9 [t(1;9)(q25;q22)], a translocation involving chromosomes 6p and 7q [t(6;7)(p25.3;q32.2)], and a terminal deletion involving chromosome 16q. The remaining cells had a normal male karyotype.
The t(6;7)(p25.3;q32.2) has been identified almost exclusively in ALK-negative anaplastic large cell lymphomas involving the DUSP22 phosphatase gene on 6p25.3 and the FRA7H fragile site on 7q32.3.<sup>1</sup> This translocation is associated with downregulation of DUSP22, which encodes a dual specificity phosphatase that regulates mitogen-activated protein kinase signaling.<sup>1</sup> Previous studies have used fluorescence in situ hybridization (FISH) to identify disruption of the DUSP22-IRF4 locus.<sup>1–4</sup> Rearrangement of the DUSP22 gene was confirmed in this case by FISH with a DUSP22 break apart probe (Fig. 5).
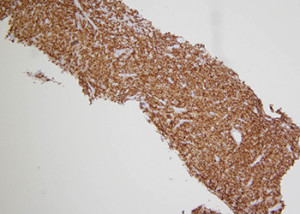
Fig. 2. The neoplastic cells expressed diffuse CD30 staining. 100× magnification.
Morphologically, DUSP22 rearranged ALK-negative anaplastic large cell lymphomas have been described as appearing similar to those that are ALK-positive with the exception that they typically demonstrate diffuse sheets of smaller hallmark cells, with prominent nuclear indentations.<sup>2</sup> When these cells are cut perpendicular to the indentation, they give the appearance of doughnut cells.<sup>2</sup> In addition, these cases also lack large pleomorphic forms that are typically described in ALK-positive and ALK-negative anaplastic large cell lymphomas.<sup>2</sup> The presence of smaller hallmark cells with doughnut cells is suggestive of a DUSP22 rearranged ALK-negative anaplastic large cell lymphoma.<sup>2</sup>
A recent multi-institutional study identified the DUSP22 rearrangement in 30 percent of the ALK-negative anaplastic large cell lymphomas they sampled.<sup>3</sup> Traditionally, patients with ALK-negative anaplastic large cell lymphoma have five-year overall survival rates inferior to those with ALK-positive anaplastic large cell lymphoma, 52 percent compared with 85 percent, respectively.<sup>3</sup> ALK-negative cases that expressed the t(6;7)(p25.3;q32.3) translocation were found to have slightly better age-adjusted five-year overall survival rates compared with those with ALK positivity, 90 percent compared with 85 percent, respectively.<sup>3</sup> Therefore, it is important to identify these DUSP22-positive patients as they have a better prognosis than traditionally once thought for ALK-negative anaplastic large cell lymphomas.

Fig. 3. The bone marrow core biopsy showing the diffuse involvement by the large neoplastic cells with increased mitotic activity and hallmark cells (black arrows). H&E staining at 500× magnification.
This same study also identified another recurrent mutation in ALK-negative anaplastic large cell lymphomas involving rearrangement of the TP53 homologue, TP63 on 3q28, in eight percent of the ALK-negative cases studied.<sup>3</sup> Patients exhibiting this mutation had age-adjusted five-year overall survival rates significantly worse than those with ALK-positive disease, 17 percent compared with 85 percent, respectively.<sup>3</sup> ALK, DUSP22, and TP63 rearrangements are mutually exclusive. Again, it is important to assess the cytogenetics in these patients as the results have a significant impact on the prognosis.
One research study has suggested an association between the DUSP22 rearrangement and the increased expression of chemokine (C-C motif) receptor 8 gene, CCR8.<sup>4</sup> The overexpression of CCR8 has been identified in primary cutaneous anaplastic large cell lymphomas and has skin-homing properties; therefore, it is postulated that it limits the extracutaneous spread of the disease.<sup>4</sup> The association between the two genes may be related to these more favorable outcomes secondary to the homing signals preventing more aggressive dissemination.<sup>4</sup> Further studies are needed to investigate these relationships and the prognostic significance of these findings.

Fig. 4. Bone marrow chromosome analysis revealed a complex karyotype that included the t(6;7)(p25.3;q32.2) resulting in DUSP22 gene rearrangement in four of 20 metaphase cells.
ALK-negative anaplastic large cell lymphoma patients treated with the standard chemotherapy regimen, which includes cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), shows variable results, with five-year overall survival rates ranging from 15 percent to 62 percent.<sup>5</sup> After induction chemotherapy, some patients receive a consolidative autologous transplantation in first complete remission, although it is difficult to determine which patients will respond to this regimen.5 The variability in overall survival rates and treatment responsiveness may be due to the genetic heterogeneity of the disease; therefore, additional studies are warranted to further examine who will benefit from more aggressive treatments.
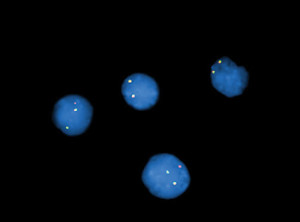
Fig. 5. Interphase fluorescence in situ hybridization analysis using a DUSP22 break apart DNA probe confirmed DUSP22 gene rearrangement (abnormal hybridization pattern consisting of one red, one green, and one fusion signal).
After the patient received six cycles of CHOEP (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone), a follow-up bone marrow study showed trilineage hematopoiesis without evidence of residual lymphoma. Concurrent cytogenetic analysis of the bone marrow revealed a normal male karyotype without evidence of the abnormal clone identified previously. The patient achieved first complete remission and received an autologous peripheral blood stem cell transplant. A follow-up PET scan 233 days status post-transplant showed no focal abnormal FDG activity that would suggest residual malignancy. At this time the patient has completed his chemotherapy regimen without recurrence of disease. We speculate that the patient will have a favorable outcome based on recent literature and the presence of the DUSP22 rearrangement. The clinical significance of additional chromosome abnormalities identified in this case with regard to prognosis is uncertain.
- Feldman A, Dogan A, Smith DI, et al. Discovery of recurrent t(6;7)(p25.3;q32.3) translocations in ALK-negative anaplastic large cell lymphomas by massively parallel genomic sequencing. Blood. 2011;117(3):915–919.
- King RL, Dao LN, McPhail ED, et al. Morphologic features of ALK-negative anaplastic large cell lymphomas with DUSP22 rearrangements. Am J Surg Pathol. 2016;40(1):36–43.
- Parrilla Castellar ER, Jaffe ES, Said JW, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014;124(9):1473–1480.
- Xing X, Flotte TJ, Law ME, et al. Expression of the chemokine receptor gene, CCR8, is associated with DUSP22 rearrangements in anaplastic large cell lymphoma. Appl Immunohistochem Mol Morphol. 2015;23(8):580–589.
- Hapgood G, Savage KJ. The biology and management of systemic anaplastic large cell lymphoma. Blood. 2015;126(1):17–25.
[hr]
Dr. Haberichter is a pathology resident, Dr. Crisan is the medical director of molecular pathology, and Dr. Micale is the medical director of the clinical cytogenomics laboratory, Department of Pathology and Laboratory Medicine, Beaumont Health, Royal Oak, Mich.