Anne Paxton
May 2023—For fluid biomarkers in the diagnosis of Alzheimer’s disease, the pace of research and related industry and regulatory developments in the past year has been brisk.
Among them: FDA de novo marketing authorization for Fujirebio’s Lumipulse G β-Amyloid Ratio (1–42/1–40) in vitro diagnostic test. FDA 510(k) clearance for Roche’s Elecsys beta-Amyloid (1–42) CSF II and Phospho-Tau (181P) CSF assays, and breakthrough device designation for its Elecsys Amyloid Plasma Panel. Publication of an international study of 40 centers that measure CSF amyloid and tau biomarkers in the clinical setting. And in January this year, the FDA’s accelerated approval of lecanemab for use in early Alzheimer’s disease.
There’s no short way to sum up the net impact. But the subplot—with fluid biomarkers possibly jockeying for position—evokes All About Eve.
CSF biomarkers were included years ago in international guidelines for the diagnosis of Alzheimer’s disease in research settings and clinical practice, and the Alzheimer’s Association’s appropriate use criteria for lumbar puncture and CSF testing in diagnosing AD were published in 2018. Blood biomarkers have not yet caught up, but they may not stay sidelined for long. They present useful testing possibilities while posing fewer drawbacks than CSF. Will CSF biomarkers gradually fade into the background, like Bette Davis’ Margo Channing? Will blood biomarkers, playing the Eve Harrington role, elbow aside CSF in the diagnosis of Alzheimer’s disease?
Leslie Shaw, PhD, professor, director of the toxicology laboratory, and director of the biomarker research laboratory, Department of Pathology and Laboratory Medicine, University of Pennsylvania Perelman School of Medicine, believes blood biomarkers—minimally invasive and for which collection is less costly—are poised to expand and perform on a par with CSF biomarkers. “I see validation and automation happening within the next two years,” Dr. Shaw predicts.
His interest in biomarkers dates back to 2003 when a colleague, neuropathologist John Trojanowski, MD, PhD, asked, “How would you like to set up a biomarker core lab for this new Alzheimer’s Disease Neuroimaging Initiative?” ADNI, now in its 19th year and fourth phase, is a study that aimed, first, to standardize Alzheimer’s disease testing methods across imaging, biofluids, and neuropsychological evaluation and, second, to establish a biobank for biofluid samples, Dr. Shaw says.
He was and remains keenly interested in standardizing methods to measure key biomarkers Aβ42 and ultimately Aβ40 in CSF, tau, and phosphorylated tau. At the time, “virtually no clinical labs provided CSF testing for clinical use. It was very much wrapped up in the research space.” There were no good tests at all in blood plasma, he says, though research was underway even then to determine whether these biomarkers could be measured reliably in plasma.

Dr. Shaw
With CSF testing now automated on widely available platforms, research on the analytical performance of CSF tests was a natural next step. Imaging is expensive and not widely available; lumbar punctures are invasive and not performed routinely in outpatients in the United States. “But in some European countries, CSF testing is routine as we speak. Europe is well ahead of the U.S. in terms of routine clinical testing of CSF,” Dr. Shaw says.
That’s partly how he came to be a coauthor of the international study on clinical reporting of results following the quantification of CSF biomarkers in Alzheimer’s disease (Delaby C, et al. Alzheimers Dement. 2022;18[10]:1868–1879).
Data were collected in 2020 from 40 laboratories in 15 countries, including the U.S., on their preanalytical, analytical, and postanalytical protocols and the reporting comments they used for the most common biochemical profiles they encountered. The three core biomarkers were Aβ1–42, t-tau, and p-tau(181), and more than 83 percent of laboratories also measured Aβ1–40 and thereafter computed the Aβ1–42/Aβ1–40 ratio. Most used similar polypropylene tubes for collection and similar storage conditions (−20°C for short term, −80°C for long term). For measurement, 88 percent used automated immunoassay analyzers.
The authors describe as a “striking finding” the wide dispersion of the cutoff values they used, “linked on the one hand to the different assays/protocols used, but also to the fact that most centers adapt the values proposed by the test providers using results from their own cohorts,” they wrote.
There was also a lot of variation in the report comments, leading the group to identify and select by consensus the most accurate and informative interpretive comments for CSF biomarker reporting in the context of Alzheimer’s disease (Table 1).
Says Dr. Shaw: “Standardization of sample preparation, calibrator accuracy, and having a reference material for calibration standardization are the kinds of things that have now been done in the CSF space and are a high-need area for plasma-based testing. Those are issues that will be presented in this summer’s Alzheimer’s Association International Conference.”
As to reporting, he says: “Cutoffs are dependent on multiple things: first, the analytical method and its accuracy and traceability to a reference material, then how to do it statistically. What population do you use? What reference standard do you use for amyloid plaque burden—the CSF testing or do you use amyloid PET? That has to be sorted out and defined, and we’re a ways away from achieving that.”
He and his coauthors wrote that the study “is an essential first step towards harmonization of the clinical reporting of the CSF biomarkers panel for the diagnosis of AD,” a framework that is adaptable to new CSF markers as they’re approved and useful in “defining reporting comments for emerging blood biomarkers of AD.”
In the United States, Dr. Shaw says, studies are underway on a related issue: communication from clinician to patient or caregiver. “It’s not going to be, ‘You’re positive for Alzheimer’s.’ Saying ‘consistent with amyloid plaque burden,’ short of knowing your tau status, doesn’t mean too much. No, it has to be more nuanced. And we have wonderful investigators who are committed to thoughtfully developing the process of communicating biomarker results to patients so that it’s done right.”
If a patient has a negative biomarker signature for amyloid plaque burden and tau tangle burden, an Alzheimer’s disease diagnosis may be excluded at that time. The patient is more likely to have other causes of their symptoms, Dr. Shaw says, possibly another neurodegenerative disease, other organic disease of the brain, or depression. “We have good tools to exclude AD. On the other hand, if someone tests positive for one or more of these biomarker tests, that can place that individual somewhere on the Alzheimer’s disease continuum over decades of time. It doesn’t exclude other diseases, though. And we know that Alzheimer’s in elderly patients is a heterogeneous disease. It’s not pure plaques and tangles.”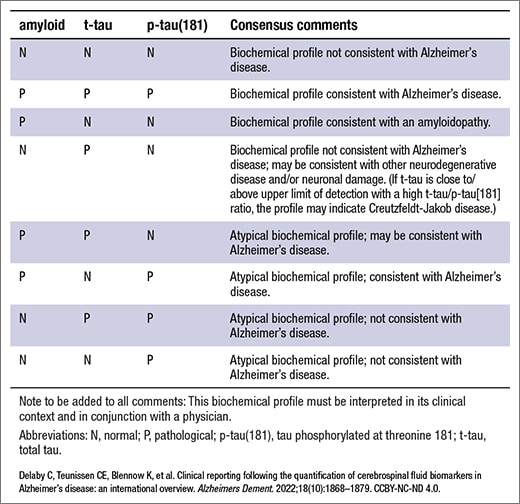
Table 1. Summary of consensus comments for interpretation of biochemical profiles of AD biomarkers in CSF
How close are clinicians to having practical and effective blood-based testing? He turns the question back. “Well, where are we with just preanalytics? Does it matter how you handle and prepare plasma?” He and colleagues studied sample preparation conditions for plasma with blood from EDTA purple-top tubes, which by general consensus, he says, is the sample of choice when it comes to plasma. “We know that for some analytes, serum works well. Not for all of them. Whereas plasma, at least for the analytes we’re interested in, works very well. We’ve got a good handle on preanalytics for plasma. We will have arrived at that a lot quicker than we arrived at standardizing blood sample collection tubes for CSF because of the fact that analytes, like Aβ especially, are very sticky to the plastics. We didn’t have the benefit of large amounts of albumin buffering out the adherence or adsorption to active sites on plastic tube walls in the instance of CSF testing.”
But the role of the tube was found to be huge in CSF testing. “There is much less what I call ‘protective’ proteins like albumin. In both serum and plasma, we have 3.5 or 4 mg/dL of albumin that protects the other proteins in that sample from things like adsorption to plastic, which plagued the CSF sample preparation for many years. But now we have very good, well-defined plastic tube types for CSF.”
In terms of methodologies for plasma, Dr. Shaw says, “say we start with Aβ 42/40 with plasma and it turns out that the study of those analytes in plasma set the stage for a lot more progress than with other analytes with 42/40. When we look at the difference in values, there’s about a 10 to 15 percent difference in the mean values. Most clinical chemists would say, Oh my goodness, what am I doing getting involved in this? But other groups, working independently using mass spec technologies, proved that we can reliably see and measure these differences between individuals in the AD cohort and the non-AD cohort.”
A Mayo clinic study also showed p-tau can be measured in plasma, he says. “So those two things: the mass spec-based testing for Aβ 42/40 and ultimately the immunoassay plasma-based testing for p-tau181” opened the floodgates to a lot more studies. An important one that Dr. Shaw is involved in is a head-to-head comparison of the different approaches to measuring these analytes. The first one of those comparisons, overseen by the Foundation for the NIH Biomarkers Consortium, together with ADNI, using ADNI plasma samples, has been published (Zicha S, et al. Alzheimers Dement. Published online July 12, 2022. doi:10.1002/alz.12697).
“We used 130 plasma samples—half from amyloid PET negatives and half from amyloid PET positives. It turns out that some of the immunoassays are working very well compared with mass spec, almost equivalently, and making it thinkable that this testing could be scalable. So this put everybody thinking this is not going to be pie in the sky; it’s going to be real and measurable and doable.” The same studies were performed for different manufacturers of p-tau181 proteoform testing and, currently, measures of p-tau217.
How good is the plasma testing compared with CSF testing? The area under the curve when CSF is compared with amyloid PET ranges from, in many studies, up to as high as 0.97, Dr. Shaw says. With plasma, the area under the curve alone is 0.85 or 0.86 up to 0.9 when the Aβ42/40 ratio is combined with a p-tau measure and the patient’s APOE ε4 genotype is included.
“I don’t want to be loose in any way, shape, or form about where the field of plasma testing is at,” he says. “But it took many decades for CSF to get to where we are today. We’re hoping to get there sooner with plasma.”
He can’t predict how soon plasma-based testing might be used in AD diagnosis. “I can’t crystal-ball that,” Dr. Shaw says. “I can say that several companies are committed to the process and that’s a very important first step. The landscape for getting FDA approval, we don’t know yet.” The likely first candidate for FDA approval is neurofilament light chain, he says.
Lecanemab and aducanumab, which are the only treatments that have been shown to slow Alzheimer’s disease progression, lend weight to the need for blood-based biomarkers. Whenever a third therapy that can remove amyloid plaque to slow AD progression becomes available, it will be pivotal in creating demand for blood biomarkers, Dr. Shaw says. The third likely prospect for approval, in his view, is Eli Lilly’s donanemab, which the FDA reviewed for accelerated approval but returned to Lilly in January this year with a request for more data because not enough patients under study had at least 12 months of exposure to the drug. Lilly plans to submit donanemab for traditional approval mid-this year.
“Once one of those targeted treatments is fully approved, and becomes reimbursable as a treatment,” Dr. Shaw says, “that is going to open the floodgates to blood-based testing demand. Because if a person is not an AD patient and they do have some form of decline, we don’t want to put them on an amyloid-lowering agent. So there’s going to be a demand for a biomarker assessment to help define who can receive the drug once it’s approved.”
Once blood biomarker testing is validated and working on automated systems, as he predicts will happen soon, “all of us want to be as ready as we can be, with respect not only to being able to perform the tests reliably, but also with respect to how to report that out. And that process means we, in the lab, engage with our clinical colleagues to say, ‘We’re thinking of introducing this testing, but we want to engage with you beforehand to iron out the issues of how we report what we find.’”

Dr. DeMarco
But it was a slow march for CSF biomarkers to make it into the clinical domain, says Dr. DeMarco, who is a coauthor of the 40-center international study of CSF clinical reporting. One reason was the analytical performance problems of the early assays. “But contemporary assays are so much better,” she says. “We have so much more knowledge about the appropriate preanalytics and improved analytics. That has launched these biomarkers into the clinical space, resulting in greater uptake.”
In the build-up to clinically implementing Alzheimer’s disease biomarkers in Canada, Dr. DeMarco launched the national IMPACT-AD study, with the goal of developing a comprehensive understanding of how AD biomarker testing impacts medical and personal decision-making and health care costs. IMPACT-AD looks at the effects of CSF testing on clinical utility, personal utility (impact on individuals and families), and health system economics (https://clinicaltrials.gov/ct2/show/NCT05002699).
The primary outcomes of the IMPACT-AD study are now coming into focus (Patel KP, et al. Clinical value of Alzheimer’s disease biomarker testing. In preparation). “In the clinical utility arm of the study, we found tremendous impact and changes in patient care based on having knowledge of the Alzheimer’s disease biomarker results,” Dr. DeMarco reports. “We found this testing improved appropriate utilization of symptomatic drug therapies and decreased the need for additional diagnostic testing, such as expensive head MRIs and CT scans, as the follow-up repeat testing usually needed to narrow down the diagnosis was no longer needed.”
“And there was a big decrease in referral for detailed neuropsychological evaluation, as physicians were more confident in their assessment of the likelihood that the patient did or did not have Alzheimer’s pathology.” This was a benefit to the patient, she says. “Our physicians told us patients can have a lot of anxiety about having to undergo detailed neuropsych testing—they are more worried about this cognitive testing than they are about having a lumbar puncture.”
Most clinicians also want to know what the patient has, not simply whether the patient has Alzheimer’s, Dr. DeMarco adds. “Alzheimer’s disease biomarkers are the ones we best understand, and that is why they are at the forefront as far as implementation in medical care.” But she also works in her research laboratory on biomarkers for Lewy body dementia, frontal temporal dementia, and other diseases that overlap phenotypically with Alzheimer’s. “We hope to be able to add to those biomarker panels” to identify when patients have other forms of dementia.
Despite the diagnostic accuracy of CSF biomarkers and amyloid PET scans, “there’s a tremendous push on accelerating the transition of blood-based biomarkers from the research space to the clinical space,” she says. “Just far above and beyond what we saw for CSF.”
“But of course, who would want to get a lumbar puncture when you could just get a simple blood test? So ease of accessibility is the core driver for blood-based biomarkers. Relatively recent analytical advancements combined with clinical study data have revealed biomarker signatures in blood for Alzheimer’s pathology that show great promise.”
That means greater investment by companies and ideally more options, Dr. DeMarco adds. “Usually the more options, the better. Competition spurs development and improved analytics, and that is something we are looking forward to in the blood space, which we didn’t have to the same degree in the CSF space.”
For all biomarkers, preanalytics remain important, she says, and the laboratory has to ensure that users of the laboratory’s testing service follow the protocols needed to achieve accuracy and precision. “The mystery has been solved, but now it is an implementation challenge to ensure that everyone using your testing service is following the proper protocol.”
She suggests that blood-based biomarker research can be classified into two broad bins. First, there are “pathology or disease-specific biomarkers, like the amyloid betas and tau proteoforms. In blood, a main challenge is detecting a ‘signal’ that may be obscured by peripheral production of related proteoforms.” Second, “you have the more general nonspecific biomarkers that are akin to a C-reactive protein test for the brain—signs of potential inflammation or damage to brain cells without specificity to a particular disease.”
Biomarkers play a key role in taking advantage of truly targeted AD therapies, Dr. DeMarco says. “With the availability of disease-modifying therapeutics that target the pathology, biomarkers become essential in identifying those individuals who would benefit from the therapy and helping doctors diagnose those people early in the disease course when they have the greatest chance to benefit from the drugs.”
Roche in July 2022 received FDA breakthrough device designation for its Elecsys Amyloid Plasma Panel, currently undergoing additional investigation. The panel measures phosphorylated tau (p-tau181) protein and apolipoprotein (APOE) E4 in human blood plasma.Corinne Fantz, PhD, chief medical partner, core lab and point of care, and vice president of medical and scientific affairs, Roche Diagnostics North America, considers the designation to be a signal from the FDA “that there’s a lot of attention to Alzheimer’s disease and clinicians need better tools to help assess more patients and get them to treatment.”
Alzheimer’s research has been underway for decades with little progress until recently, Dr. Fantz notes. “There’s still no silver bullet to cure Alzheimer’s, but some of the anti-amyloid drugs have demonstrated the ability to slow disease progression. The FDA has insight and knows what drugs are coming to the market soon. These treatments are showing promise in clearing amyloid from the brains of people who have plaque build-up, but the problem is determining who is eligible for treatment. We need tools like these biomarkers as a way of getting more people into that treatment pathway.”
One way to do that is with the CSF test. “A PET scan is another way, but not every hospital has PET scanners. When the blood-based tests become available on automated platforms, like a Roche Cobas immunoanalyzer, you could run the tests at any hospital with access to that instrument, then confirm amyloid pathology with a PET scan or a test that correlates to a PET scan, the CSF ratio.”
The blood-based biomarker is not a diagnostic test, she says, “but it can help exclude other causes of dementia,” so it can help streamline the path to therapy.

Dr. Fantz
The biomarkers are helping in many clinical trials of therapies to pinpoint the disease stages, Dr. Fantz says. “Often by the time you have symptoms, the disease progress has already been happening for many years, and there are a lot of questions on how effective the therapies are. Because by the time you’ve had symptoms, the pathology has already been set in motion, but there is hope that earlier diagnosis will yield more effective treatments.”
The biomarker assays, as LDTs for the most part, have not been evaluated with the same level of rigor that is typical for an in vitro diagnostic assay, which would have had FDA reviews, she says. Defining the conditions for collection, storage, and analytical techniques is critical, she says, to ensure the results are meaningful for clinical decision-making. The advantage of having the breakthrough designation is that it recognizes that these markers can make a difference for patients who are on their journey to a diagnosis, she says. “There is an opportunity for patients to get enrolled in these trials, and we can study disease progression to potentially find earlier and better therapies in the future.”
CSF tests came to market earlier than blood biomarkers, she says, because the amount of protein in the CSF versus what’s in the blood is much higher and doesn’t require the sensitivity to detect the lower concentrations typically found in blood. But Roche, she reports, continues to work on a full pipeline of neurology biomarkers and is aiming by 2025 to have significant new AD biomarker approvals to announce.
“It’s important for labs to know that these therapies are coming and that there will be demand for testing,” she says. “Typically, neurologists have not been high users of the lab. So laboratorians may want to reach out and let them know their systems and capabilities and what they can offer. That can help these clinicians understand what can be expected from a diagnostic perspective.”
Dr. Shaw is upbeat about the future of both CSF and blood biomarkers. “We can definitely look forward to the prospect for CSF testing of two available, highly automated methods that are very good. They’ve been tested and they are sound. But there’s unlikely to be a hugely high demand for them because of the known limitations of lumbar puncture in an outpatient setting.”
Very soon, he says, “we can look forward to the day when these blood-based biomarker tests are validated and ready for reliable analysis on automated systems.” Also playing an important role will be the full clinical evaluation that the memory disorders specialists perform with the patient and involving the caregiver. “Whenever we consider testing,” Dr. Shaw says, “it always needs to be in the context of a lot of other parameters.”
Anne Paxton is a writer and attorney in Seattle.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
