CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from University Hospitals Cleveland Medical Center and Case Western Reserve University School of Medicine. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Catherine M. Stefaniuk, DO
Daniel D. Rhoads, MD
Michael R. Jacobs, MB BCh, PhD
September 2020—A 64-year-old male presented with worsening shortness of breath, dry cough, and bilateral leg edema. He had a history of diabetes mellitus type two, hypertension, seropositive rheumatoid arthritis, and tobacco and alcohol abuse. CT scan demonstrated bilateral pleural effusions, pulmonary edema, subsegmental atelectasis, mildly enlarged hilar lymph nodes, mild cardiomegaly with a small pericardial effusion, and liver cirrhosis with a liver nodule. A hepatitis panel demonstrated positive serology for hepatitis C virus infection. Ultrasound-guided core biopsies of liver nodule demonstrated hepatocellular carcinoma. Endoscopy demonstrated grade one esophageal varices and a gastric ulcer. Biopsy of the gastric ulcer demonstrated acute and chronic inflammation with presence of Helicobacter pylori.
The patient was started on a proton pump inhibitor, amoxicillin, and clarithromycin. Transthoracic echocardiogram noted a 7.6-mm lesion on the aortic valve. Transesophageal echocardiogram demonstrated aortic valve leaflets to be severely thickened with reduced systolic excursion and oscillating vegetations on two cusps suggestive of endocarditis. Patient denied history of worsening arthralgias, nausea, vomiting, weight loss, swimming, travel, hunting, dental procedures, sick contacts, or animal contacts. He did admit to a two- to three-month history of night sweats. Multiple blood cultures were negative. Patient was taken to surgery to replace the aortic valve; vegetations were present on all three aortic valve leaflets, with destruction of the right coronary and noncoronary cusps. The excised aortic valve was submitted for culture, histology, and molecular analysis.
Details of the pathology. Grossly, aortic valve leaflets were fragmented with a fibrotic thickened surface covered by vegetations and calcifications (Fig. 1). Microscopic examination demonstrated multiple foci of acute and chronic inflammation of valve leaflets, with cusp destruction and marked infiltration by basophilic foamy macrophages and multinucleated giant cells, admixed with acute inflammation in necrotic debris (Figs. 2 and 3). Myxoid degenerative changes with dystrophic calcifications and hemosiderin-laden macrophages were also present.
Grocott’s methenamine silver stain (GMS) demonstrated thin bacilli within macrophages in areas of acute inflammation and necrotic debris (Fig. 4). Periodic acid-Schiff stain (PAS) revealed macrophages with abundant PAS-positive inclusions (Fig. 5). Both Gram and acid-fast stains (Ziehl-Neelsen stain) were negative. All cultures were also negative. These findings favored Tropheryma whipplei infection. Since PAS and GMS are nonspecific, molecular analysis was sought to prove this diagnosis.
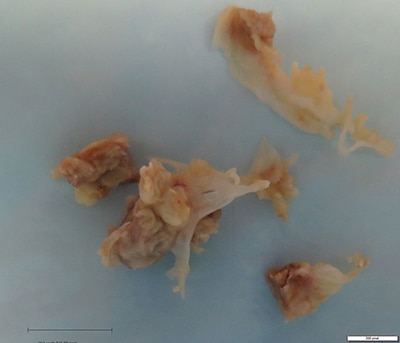
Fig. 1. Aortic valve leaflets with vegetations present (black bar = 1 cm).
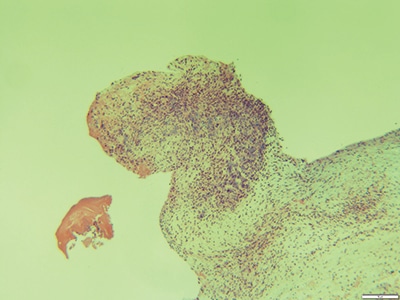
Fig. 2. Aortic valve vegetation with the presence of abundant macrophages with a basophilic hue (2×).
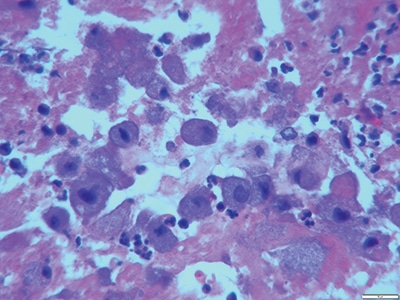
Fig. 3. Hemotoxylin and eosin stain: numerous macrophages with abundant basophilic foamy cytoplasm (40×).
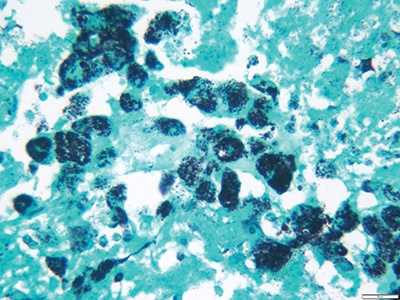
Fig. 4. Grocott’s methenamine silver stain: macrophages staining positive with T. whipplei bacilli cell wall material (40×).

Fig. 5. Periodic acid-Schiff stain: macrophages staining positive with T. whipplei bacilli cell wall material (40×).
Molecular analysis. Unpreserved valve leaflets were sent to the University of Washington Department of Laboratory Medicine for molecular analysis. DNA was extracted from unpreserved valve leaflet tissue and used as a template for amplification by universal primers complementary to conserved regions of the 16S rRNA gene. PCR products were purified and sequenced to confirm the presence of the 16S rRNA gene product demonstrating Tropheryma whipplei’s 16S rRNA gene.
Clinical impact. The patient had endocarditis due to Tropheryma whipplei. He was started on penicillin G and then switched to trimethoprim-sulfamethoxazole. He presented in a complicated setting of rheumatoid arthritis, immunosuppression, congestive heart failure, liver cirrhosis, HCV, hepatocellular carcinoma, and Helicobactor pylori infection. If the patient had not had an echocardiogram performed because of his heart failure, his diagnosis of endocarditis would have been delayed because blood cultures were negative.
Conclusions. Tropheryma whipplei is a rare cause of endocarditis and is a difficult organism to identify since it does not grow in culture media.1-3 Whipple disease can present as isolated organ manifestations (migrating oligo/polyarthritis, acute gastroenteritis, pulmonary disease, endocarditis, or central nervous system involvement) or as the classical (systemic) form with aggravated diarrhea, malabsorption, weight loss, abdominal pain, migratory arthralgias of the large joints, and lymphadenopathy. Determining the causative agent of culture-negative endocarditis is extremely beneficial for patients. Whipple disease is fatal if untreated and requires close follow-up for signs of central nervous system involvement and possible relapse of the disease. Patients with Tropheryma whipplei are treated for a year or longer with antimicrobials since rates of relapse can be high. Initial therapy is intravenous ceftriaxone or penicillin G for two weeks with maintenance therapy of trimethoprim-sulfamethoxazole for one to two years.4,5 More recent proposed therapy is oral doxycycline combined with hydroxychloroquine for 12 to 18 months.1 Sequencing for 16S rRNA has been extremely beneficial in cases where bacterial infections are expected but cultures have been negative. The 16S rRNA gene has both conserved and variable regions; the conserved regions enable primers to bind to and amplify the variable regions of the gene.6-8,10,11 The species-specific variable regions are used for identification by sequence alignment with the public database at the National Center for Biotechnology Information (BLAST).6-8,10 Next-generation sequencing typically is used with either the Illumina or Ion Torrent platforms.
- Dolmans RA, Boel CH, Lacle MM, Kusters JG. Clinical manifestations, treatment, and diagnosis of Tropheryma whipplei infections. Clin Microbiol Rev. 2017;30(2):529–555.
- Dutly F, Altwegg M. Whipple’s disease and “Tropheryma whippelii.” Clin Microbiol Rev. 2001;14(3):561–583.
- Fenollar F, Célard M, Lagier JC, Lepidi H, Fournier PE, Raoult D. Tropheryma whipplei endocarditis. Emerg Infect Dis. 2013;19(11):1721–1730.
- Schneider T, Moos V, Loddenkemper C, Marth T, Fenollar F, Raoult D. Whipple’s disease: new aspects of pathogenesis and treatment. Lancet Infect Dis. 2008;8(3):179–190.
- Pierce D, Calkins BC, Thornton K. Infectious endocarditis: diagnosis and treatment. Am Fam Physician. 2012;85(10):981–986.
- Marchesi R, Sato T, Weightman AJ, et al. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol. 1998;64(2):795–799.
- Shrestha N, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg. 2015;99(1):33–37.
- Clarridge JE 3rd. Impact of 16S rRNA gene sequencing analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev. 2004;17(4):840–862.
- Biagi F, Badulli C, Feurle GE, et al. Cytokine genetic profile in Whipple’s disease. Eur J Clin Microbiol Infect Dis. 2012;31(11):3145–3150.
- Salipante SJ, Kawashima T, Rosenthal C, et al. Performance comparison of Illumina and Ion Torrent next-generation sequencing platforms for 16S rRNA-based bacterial community profiling. Appl Environ Microbiol. 2014;80(24):7583–7591. Published correction appears in Appl Environ Microbiol. 2016;82(14):4453.
- Salipante SJ, Sengupta DJ, Rosenthal C, et al. Rapid 16S rRNA next-generation sequencing of polymicrobial clinical samples for diagnosis of complex bacterial infections. PLoS One. 2013;8(5):e65226.
Dr. Stefaniuk is assistant professor and assistant clinical laboratory director, University of Cincinnati College of Medicine and University of Cincinnati Medical Center. Dr. Rhoads is assistant professor and section head of microbiology, Cleveland Clinic. Dr. Jacobs is professor emeritus, Case Western Reserve University School of Medicine and University Hospitals Cleveland Medical Center.
Test yourself
- Here are three questions taken from the case report.
- Answers
1. What is the benefit of 16S rRNA sequencing?
a. It is able to sequence organisms that do not grow in culture.
b. It is able to sequence organisms that grow in culture.
c. It is able to sequence fastidious and intracellular organisms.
d. All of the above.
2. Whipple disease is preferentially seen in which patient population:
a. Caucasian American males
b. African American males
c. Asian American males
d. Native American males
3. Broad-range PCR primers that are used to sequence the 16S rRNA gene target:
a. the conserved regions of the 16S rDNA.
b. the hypervariable regions of the 16S rDNA.
c. the promoter regions of the 16S rDNA.
d. the untranslated region of 16S rDNA.
1. What is the benefit of 16S rRNA sequencing?
a. It is able to sequence organisms that do not grow in culture.
b. It is able to sequence organisms that grow in culture.
c. It is able to sequence fastidious and intracellular organisms.
d. All of the above. Explanation: 16S rDNA sequencing can detect the causative bacterial agent of infection directly from normally sterile tissue or fluids. It is beneficial when a specimen is available that is thought to be infected but a causative agent has not been definitively identified by culture.
2. Whipple disease is preferentially seen in which patient population:
a. Caucasian American males. Explanation: The typical patient is a Caucasian middle-aged male, though Whipple disease can be rarely seen in females and patients of other ethnic backgrounds. There is an association with HLA DRB1*13 and DQB1*06.
b. African American males
c. Asian American males
d. Native American males
3. Broad-range PCR primers that are used to sequence the 16S rRNA gene target:
a. the conserved regions of the 16S rDNA. Explanation: The PCR primers target and sequence the highly conserved regions of the 16s rRNA gene which lead to identification of the organism.
b. the hypervariable regions of the 16S rDNA.
c. the promoter regions of the 16S rDNA.
d. the untranslated region of 16S rDNA.