Karen Lusky
April 2016—In his CAP ’15 presentation last fall, David Bostwick, MD, MBA, referred to intraductal carcinoma of the prostate as “sort of the rage right now in the urologic pathology field.”
“The problem is that it has multiple different definitions, and interobserver agreement with it is moderate at best,” said Dr. Bostwick, medical director of Granger Diagnostics in Richmond, Va. Even when pathologists can agree on an IDC diagnosis, he said, they aren’t on the same page about treatment.

Dr. Zhou
“By definition, intraductal carcinoma itself is not invasive,” Ming Zhou, MD, PhD, said in a CAP TODAY interview. “In most cases, intraductal carcinoma represents the spread of invasive cancer into the preexisting prostate ducts and glands. So the intraductal carcinoma glands will have retained their basal cells,” said Dr. Zhou, a professor of pathology and urology and director of surgical pathology and urologic pathology at New York University Medical Center Tisch Hospital in New York City.
Laurence Klotz, MD, a urologic oncologist at Sunnybrook Health Sciences Centre and a professor of surgery at the University of Toronto, said intraductal carcinoma “is really a sign that there’s aggressive disease elsewhere. It’s also quite rare so that’s part of its strength. When you find it, you have to take it seriously.”
How rare? Dr. Zhou and colleagues found that the prevalence of IDC in prospectively collected prostate biopsies was about 2.8 percent (Watts K, et al. Histopathology. 2013;63[4]:574–579), which means, he said, that about three out of 100 biopsies may have a lesion that could be diagnosed as IDC. “But most of these intraductal carcinomas are associated with invasive cancer in the same biopsy. Only about one-tenth of those IDCs have so-called isolated intraductal carcinoma without invasive carcinoma. So it’s very rare.” Before diagnosing isolated IDC, pathologists have to be sure they haven’t overlooked invasive cancer, Dr. Zhou cautions.
The differential diagnosis for IDC, he said, includes high-grade PIN, ductal carcinoma of the prostate, urothelial carcinoma involving the prostate, and invasive cribriform cancer. “But the most important differential diagnosis is between high-grade PIN and IDC.”
Dr. Zhou noted that the National Comprehensive Cancer Network guideline does not advise repeating a biopsy within the first year of a high-grade PIN diagnosis when the high-grade PIN involves a single core on a standard 12-core biopsy. “In contrast, IDC is almost always associated with high-grade and [high]-volume cancer,” he said. “Therefore, a diagnosis of IDC without concomitant cancer warrants immediate biopsy.” Some experts recommend radical prostatectomy or radiation therapy.
In very rare cases, IDC is considered to be a precursor lesion just like high-grade PIN, Dr. Zhou said. “There is a gray zone between the two because you’re talking about a continuous spectrum of moving from a relatively low-grade proliferation to a high-grade proliferation.” Moving along this spectrum, “in the IDC, you typically see an expansile, complex, cribriform, and solid proliferation. The cytological atypia is much more pronounced” than in high-grade PIN.
Kenneth Iczkowski, MD, an associate professor of pathology at Medical College of Wisconsin, said if the lesion has marked enough cytological atypia and is distending the duct space it is in, those two features are most influential in diagnosing IDC. “Also, if there’s necrosis in the lumen of the gland or the cellularity of the cribriform proliferation is dense, those findings support IDC.”
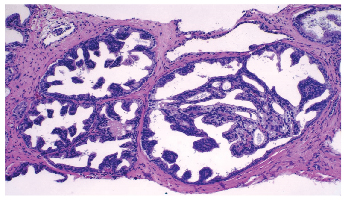
HGPIN vs. IDC?
To determine how often urologic pathologists agreed that a lesion was IDC, Dr. Iczkowski and Dr. Bostwick asked 39 urologic pathologists to examine photomicrographs of atypical duct proliferations (Iczkowski KA, et al. Ann Diagn Pathol. 2014;18[6]:333–342). The selected cases included IDC and two mimics: high-grade PIN and invasive cribriform/ductal carcinoma. “There was 70% overall agreement with HGPIN, 43% with IDC, and 73% with invasive carcinoma,” the authors wrote.
“When the 19 of 38 images that attained consensus for HGPIN or invasive carcinoma were removed from consideration, lack of IDC consensus was most often attributable to only loose cribriform growth (5/19), central nuclear maturation (5/19), or comedonecrosis (3/19),” they reported in the article.
“It turns out,” Dr. Bostwick said, “that most of the time when you see something in a larger acinar structure, it’s not going to have loose cribriform growth. It’s going to be solid.”
Dr. Bostwick showed CAP ’15 attendees an image and asked whether it was high-grade PIN or intraductal carcinoma. “It’s big. It has cribriform and micropapillary growth. It’s probably one or the other,” he said. (See “HGPIN vs. IDC?” above.)
In providing the answer (IDC), Dr. Bostwick said a study reported in the February 2015 issue of the American Journal of Surgical Pathology (of which Dr. Zhou is a coauthor) showed that the combination of ERG staining and loss of PTEN staining could make the difference definitively in almost all cases. “While they aren’t perfect, they are very good in a lot of cases,” he said. The image he showed of IDC was a lesion that had alterations for both of those markers.
The authors report that 61 percent of biopsies (20/33) that contained isolated IDC demonstrated PTEN loss. Ten out of 33 (30 percent) expressed ERG. “Of the borderline intraductal proliferations, 52% (11/21) showed PTEN loss and 27% (4/15) expressed ERG,” they wrote. No PTEN loss or ERG expression was seen in the 19 PIN cases. They noted that in needle biopsies, PTEN loss is rare in high-grade PIN but common in IDC identified morphologically (Morais CL, et al. Am J Surg Pathol. 2015;
39[2]:169–178).
“Prior studies,” the authors wrote in the article, “have shown ERG expression in up to 20% of PIN cases; however, it is more commonly seen in PIN adjacent to invasive cancer or in isolated PIN diagnosed on needle biopsies from patients with a subsequent diagnosis of invasive cancer.”
Dr. Iczkowski noted that a study reported in The Prostate recommends using PTEN to differentiate high-grade PIN from IDC of the prostate (Torabi-Nezhad S, et al. Prostate. 2016;76[4]:394–401). He said PTEN and ERG can be evaluated by using FISH or by immunostains.
“PTEN is important,” Dr. Bostwick said, “because it’s a tumor or cancer suppressor gene so you basically want it turned on.”
As for clinical management, Dr. Bostwick’s view is that IDC warrants definitive therapy because he’s never seen a case of it that wasn’t associated with cancer at prostatectomy.
Dr. Zhou said he would not recommend definitive therapy for IDC without concomitant cancer in the biopsy but would “clearly communicate” to the urologist the significance of the finding and recommend an immediate repeat biopsy. At his institution, urologists use MRI-targeted biopsies in such cases. If the urologists do not see a suspicious lesion on the MRI, they still perform a standard 12-core biopsy.
Dr. Iczkowski reports that in his survey of 39 urologic pathologists, 59 percent said that whether definitive therapy for isolated IDC is indicated depends on clinicopathologic factors. The remainder of the pathologists were split evenly between saying, yes, it is an indication for definitive therapy, and, no, it warrants close follow-up and an immediate repeat biopsy but not definitive therapy.
Dr. Bostwick shared those survey findings with pathologists in the audience and polled them on their opinions, noting they were divided very much like the urologic pathologists in the survey. He predicts the availability of more objective measures in the near future. “I think this is one of those areas of pathology that will give way to molecular testing,” he said, “and then we will have a definitive answer on this.”
[hr]
Karen Lusky is a writer in Brentwood, Tenn.