Charna Albert
March 2022—Papillary and fibroepithelial lesions of the breast were the focus of a talk given by Xiaoxian (Bill) Li, MD, PhD, in a CAP21 session on common but challenging diagnoses in breast core biopsies.
Dr. Li, associate professor of pathology and director of breast pathology and of the immunohistochemistry laboratory at Emory University, began with the case of a 47-year-old woman who presented with a right breast solid mass of 1.4 cm with indistinct margins. One core biopsy shows intraductal proliferation with fibrovascular cores, “a typical papilloma,” he said. In another core of the same biopsy, under low power, small glands with a pseudo-infiltrative pattern can be seen. “So this core biopsy is perfectly correlated with the imaging impression.” At high magnification, myoepithelial cells can be seen within some glands (Figs. 1–3). And p63 staining reveals myoepithelial cells throughout the lesion. “So this is a papilloma with a pseudo-infiltrative pattern. It’s a benign lesion.” The day after diagnosis a physician calls to say the patient has comorbidities and doesn’t want surgery.
“The question is how to manage papillomas without atypia diagnosed on core biopsies,” Dr. Li continued, noting several important studies (for example: Pareja F, et al. Cancer. 2016;122[18]:2819–2827; Swapp RE, et al. Ann Surg Oncol. 2013;20[6]:1900–1905) and sharing Emory’s experience. The data from a retrospective review he and others at Emory conducted suggest that observation, rather than excision, is appropriate for radiologic-pathologic concordant benign intraductal papillomas (Li X, et al. Clin Imaging. 2020;60[1]:67–74). Dr. Li and his coauthors reviewed 188 IDP cases—none of which had atypia or concurrent ipsilateral malignancy on core biopsy—to determine the upgrade rate at surgical excision. All patients underwent surgery. Of the 188 cases, one was upgraded to a 9-mm invasive ductal carcinoma away from the papilloma, and another to a 3-mm ductal carcinoma in situ away from the papilloma. “We felt both cases were incidental findings of carcinoma on excision,” Dr. Li said.
This study and others led to biweekly high-risk breast lesion conferences at Emory, attended by breast pathologists, breast imagers, and breast surgeons. Clinical and imaging follow-up at six-month intervals was recommended for all benign papillomas with concordant pathology-radiology findings. “And we recommended surgery for all atypical papillomas because they have a high upgrade rate to invasive carcinoma or ductal carcinoma in situ,” Dr. Li said.
Dr. Li and colleagues reported their experience from the high-risk breast lesion conferences in 2020 (Ma Z, et al. Breast Cancer Res Treat. 2020;183[3]:577–584). One hundred fifty consecutive core-biopsy-diagnosed papilloma cases had been reviewed prospectively to determine whether surgical excision was necessary. One hundred twelve were benign; 17 were involved by atypical ductal hyperplasia; six had atypical ductal hyperplasia in adjacent tissue but not involving the papilloma; two were involved by atypical lobular hyperplasia; and five had atypical lobular hyperplasia in adjacent tissue. (Six were excluded due to lack of first imaging follow-up until analysis, and two were pathology-radiology discordant.)
Though follow-up was recommended for all the benign papillomas, 39 of the 112 patients opted for surgery. “And there was a zero percent upgrade rate in those 39 cases to invasive carcinoma or ductal carcinoma in situ,” Dr. Li said. The remaining patients with benign papillomas did not have disease progression during follow-up (185–1,279 days, mean 574). Fifteen of the 17 atypical papillomas were excised as recommended, with four (26.7 percent) upgraded to carcinoma.
Shortly after he and colleagues reported their prospective experience from their high-risk lesion conferences, Nakhlis F, et al., reported the results of their prospective multi-institutional trial to determine the upgrade rate to invasive cancer or DCIS at excision for intraductal papilloma without atypia on core biopsy (Nakhlis F, et al. Ann Surg Oncol. 2021;28[5]:2573–2578). Of 116 patients studied, one case was upgraded to a 3-mm low-grade DCIS, and another to a multi-foci atypical ductal hyperplasia bordering DCIS, per local review. “But by central review,” Dr. Li said, “both cases had atypical ductal hyperplasia only. So if we take the diagnosis by central review, there was no upgrade rate of these 116 benign papillomas.” Furthermore, central pathology review confirmed intraductal papilloma without atypia in only 85 of the 116 cases, and both locally upgraded cases were among them. Of the remaining cases, two were atypical, eight had atypical ductal hyperplasia in adjacent breast tissue, and 21 had a variety of benign alterations, some of which are mimics of papilloma.
In Fig. 4 is a benign lesion that can mimic papilloma—apocrine metaplasia with papillary proliferation. “I would not call this papilloma. This would not show papilloma features on imaging. So you want to have some threshold for what you call papilloma,” Dr. Li said.
In managing papilloma without atypia diagnosed on core biopsy, Dr. Li summed up, patients can be followed without excision if pathologists are confident in the diagnosis and imaging findings are concordant. “As we know, we sometimes get really tiny biopsies. If you don’t feel comfortable calling it a benign papilloma, you can always recommend re-biopsy or excision.” All atypical papillomas should be excised.
The second case Dr. Li shared was that of a 47-year-old woman with a 1.4-cm intraductal right breast mass. In the core biopsy, “you can see intraductal proliferation with some fibrovascular cores, and this one has prominent epithelial proliferation,” he said (Fig. 5). Under high magnification, most of the epithelial proliferation has features of usual ductal hyperplasia (UDH) (Fig. 6). The top center-right area of the biopsy, however, is suspicious for atypical ductal hyperplasia (ADH), and the top center-left area shows features between UDH and ADH. “So in this case, IHC will be helpful to differentiate UDH from ADH.”
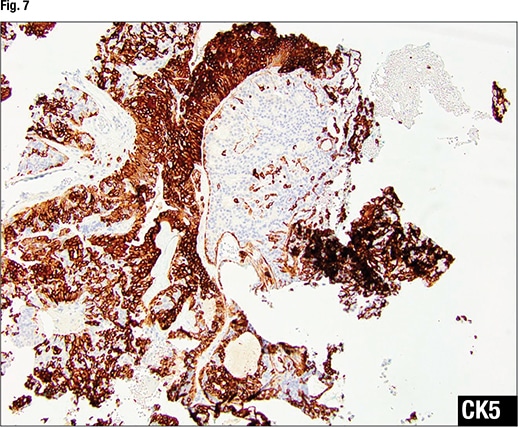
In Fig. 7 there is no CK5 staining in the area of ADH, “indicating this is a monoclonal proliferation.” In the area of UDH, “there’s a strong yet mosaic staining pattern of CK5, indicating a polyclonal proliferation.” ER staining in the area of ADH (Fig. 8) shows diffuse strong staining, indicating a monoclonal proliferation. In the area of UDH, the ER staining is focal with different intensity—some cells weak, others strong—indicating a polyclonal proliferation.
“The majority of people would diagnose this as a papilloma with ADH, but how can you differentiate papilloma with ADH versus papilloma with DCIS?” Dr. Li asked.
An area of atypical epithelial proliferation of 3 mm or larger is a papilloma with DCIS, per Page criteria, and an area of atypical epithelial proliferation smaller than 3 mm is a papilloma with ADH. If the papilloma is more than 30 percent involved ADH, per Tavassoli, it’s papilloma with DCIS, and if less, it’s papilloma with ADH. Schnitt and others, Dr. Li said, may call papilloma with DCIS when the atypical proliferation within the papilloma shows all the combined architectural and cytological features of DCIS, regardless of its extent.
Atypical proliferation within papilloma is associated with higher cancer risk, Dr. Li said, citing a 2003 study that’s “still one of the best to investigate atypical proliferation within papilloma.” The study (MacGrogan G, et al. Virchows Arch. 2003;443[5]:609–617) retrospectively analyzed 119 central intraductal papillomas presenting with ADH or florid UDH. Of the 22 patients who had papillomas with florid hyperplasia, 4.5 percent had cancer during follow-up, Dr. Li said. The 24 cases classified as atypical and the 33 classified as carcinoma arising in a papilloma had higher rates of developing cancer—12.5 percent and 15.2 percent, respectively.
In the cases of atypical papilloma where there is no atypical proliferation in the surrounding breast tissue, none of the patients had cancer in their follow-up. “However, when there was atypical proliferation in the surrounding breast tissue, three of the 17 patients had cancer during follow-up,” Dr. Li said. “When the papilloma is involved by DCIS and there’s no atypical proliferation in the surrounding breast tissue, none of the 15 patients had cancer during follow-up. However, when there was atypical proliferation in the surrounding breast tissue, five of the 17 had cancer during follow-up.” This study indicates, he added, that atypical proliferation or DCIS in the surrounding breast tissue is more important in predicting cancer risk.
When the atypical proliferation is confined within the papilloma, he said, patients have an excellent prognosis when the papilloma is excised with clear margins. “So I would be cautious to call papillomas involved by DCIS if atypical proliferation is confined within the papilloma,” Dr. Li said, noting the DCIS diagnosis may lead to more treatment.
Dr. Li turned next to papillary carcinomas in core biopsy. Papillary carcinoma, he said, is an umbrella term used in core biopsies that includes papilloma with DCIS, intraductal papillary carcinoma (papillary DCIS), encapsulated papillary carcinoma, and solid papillary carcinoma. To determine if a more accurate diagnosis on core biopsy would have clinical implications, he and colleagues conducted a 41-case study of papillary carcinoma, all of which were diagnosed as DCIS or invasive carcinoma on excision. “We examined how many cases were upgraded to invasive carcinoma to see whether we need to give a more accurate diagnosis on core biopsy,” Dr. Li explained. Based on morphology and staining, on core biopsy they diagnosed 29 cases as papillary DCIS, 10 of which were upgraded to invasive on excision (34 percent). Six were diagnosed as solid papillary carcinoma, four of which were upgraded (67 percent); and four were diagnosed as papilloma with DCIS, one of which was upgraded (25 percent). From these results, they concluded “a more accurate diagnosis of papillary carcinoma on core biopsy has no clinical implication.”
No definite invasion should be identified in a diagnosis of papillary carcinoma, Dr. Li said. “If you can see invasive carcinoma, call it invasive carcinoma.” More accurate classification of papillary carcinoma without invasion can be deferred to excision, he said, “because treatment certainly will be the same.”
Fibroepithelial lesions, Dr. Li said, are a biphasic proliferation of stromal and epithelial components classified generally as fibroadenoma or phyllodes tumor. Hamartoma is a rare fibroepithelial lesion, as is periductal stromal tumor, which is now classified as phyllodes tumor.
Sixty to 75 percent of phyllodes tumors are benign, with a 10 to 17 percent recurrence rate and no metastatic potential. Fifteen to 26 percent are borderline; they have a 14 to 25 percent recurrence rate and virtually no metastatic potential. And eight to 20 percent of phyllodes tumors are malignant, with a 23 to 30 percent recurrence rate and low metastatic potential.
Classic-type fibroadenoma morphology is straightforward, “with a stromal cellularity similar to the perilobular stroma in the surrounding benign breast tissue,” he said. But some may show unusual features. They may be involved by atypical epithelial proliferation, carcinoma in situ, or invasive carcinoma. And some have myxoid changes in the stromal component, he said, noting myxoid fibroadenomas are sometimes associated with Carney complex, so for this he recommends clinical correlation for Carney complex.
Rarely, fibroadenomas may have a focal and permeable border, Dr. Li said. They also may be involved by pseudoangiomatous stromal hyperplasia or smooth muscle differentiation. Typically, they do not contain adipose tissue, but infrequently they can have lipomatous metaplasia. Relatively large fibroadenomas, in particular, he noted, can have focal infarction. Some fibroadenomas have more stromal proliferation; at the other end of the spectrum there is more tubular proliferation.
“The problem we often face is how to differentiate cellular fibroadenomas from phyllodes tumors in core biopsies,” he said. Both have well-demarcated borders. Cellular fibroadenomas often have increased stromal cellularity; benign and borderline phyllodes tumors can have mild to moderately increased stroma. Neither fibroadenomas nor benign phyllodes tumors have severe stromal atypia, and both have low mitotic count (borderline and malignant phyllodes have a higher mitotic count; 5–9/10 HPF and ≥ 10/10 HPF, respectively). And neither fibroadenomas nor benign phyllodes tumors have stromal overgrowth or malignant heterologous components (malignant phyllodes tumors have stromal overgrowth and may have malignant heterologous components).
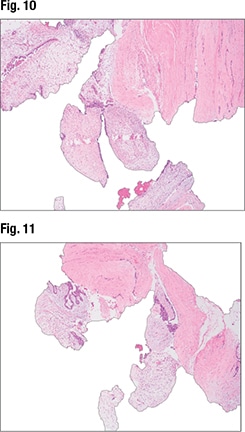
The third case Dr. Li presented is that of a 50-year-old female who presented with a 12-cm well-circumscribed breast mass. In the core biopsy, “we can see biphasic proliferation with epithelial proliferations and stromal proliferation. The biopsy is kind of fragmented,” and there’s some stromal heterogeneity in terms of the cellularity, with some areas showing more stromal cellularity and others less (Fig. 9). At higher power (Fig. 10), intratumoral heterogeneity can be seen. In Fig. 11, tissue fragmentation can be seen. Under higher magnification (Fig. 12), “there’s no severe cytologic atypia and virtually no mitosis in the stromal components.”

The lesion was initially diagnosed as a fibroadenoma with myxoid changes. On excision it looked like what was seen in the core biopsies: biphasic proliferation with stromal and epithelial components, some intratumoral heterogeneity, and some areas with more stromal cellularity and others with less. Under high magnification the background stroma are seen to have focal myxoid changes, but there’s no severe cytologic atypia. In other areas of the tumor, however, “we see stromal components without epithelial components. So this is stromal overgrowth. And some areas show increased mitosis.”
Six months later, “this patient had metastasis to the lung,” he said. The true diagnosis was malignant phyllodes tumor.
“So what features in a core biopsy are not typical of a fibroadenoma?” Large tumor size is one. Intratumoral stromal heterogeneity is another, as is tissue fragmentation, which is sometimes associated with phyllodes tumor. Several studies have noted the morphological features in core biopsy that are associated with phyllodes tumor diagnosis in excision (in addition to fragmentation), he said: stromal overgrowth, ≥ 2 mitoses/10 HPF, marked stromal hypercellularity, stromal pleomorphism, heterogeneity, infiltrating border, adipose tissue with stroma, large tumor size, and age ≥ 50 years.
“Of all these features, be careful of stromal pleomorphism,” he said. Stromal giant cells are benign reactive stromal cells that can be seen in fibroepithelial and other breast lesions (and lesions from other organs). Studies have found they do not increase the risk of recurrence of fibroepithelial lesions. They are generally negative for cytokeratin staining and mitotically inactive. “However, you can see occasional mitosis of these stromal giant cells. So be careful with the stromal giant cells,” Dr. Li cautioned. “Do not overcall these lesions.”
“It’s okay to call fibroepithelial lesion and recommend excision if you’re not confident to differentiate fibroadenoma from phyllodes tumor. But if the lesion shows all the classic features of a fibroadenoma, call fibroadenoma. Because if you call every lesion a fibroepithelial lesion, that may result in unnecessary surgeries.” And differentiating cellular fibroadenoma from phyllodes tumor can be difficult even in surgical specimens, he noted.

Lawton TJ, et al., reported significant interobserver variability even among pathologists who specialize in breast pathology in distinguishing between cellular fibroadenomas and phyllodes tumors. In only two of 21 selected cases of fibroepithelial lesions sent in consultation that were challenging to classify as fibroadenoma or phyllodes tumor was there uniform agreement (Lawton TJ, et al. Int J Surg Pathol. 2014;22[8]:695–698).
Two key features differentiate cellular fibroadenoma from benign phyllodes tumor, according to consensus opinion: a well-developed leaf-like structure (Fig. 13) and stromal cellularity. The key feature is the former because there can be overlap with the latter.
Cellular fibroadenomas, classic-type fibroadenomas, and benign phyllodes tumors harbor MED12 and RARA mutations, Dr. Li said. TERT promoter mutations occur more frequently in benign phyllodes tumor than in cellular and classic-type fibroadenoma. “So there could be progression from fibroadenoma to phyllodes tumor,” Dr. Li said, when a fibroadenoma is hit by a TERT promoter mutation.

The recurrence rate of benign phyllodes tumors varies from study to study, Dr. Li noted. “The question we usually face in practice is, ‘Do these patients need wide margins?’” In one multicenter study that evaluated the margin status to recurrence rate in 550 phyllodes tumors, 1.7 percent of benign phyllodes tumors with positive margins recurred. When there was a negative but narrow margin (< 2 mm), 2.7 percent recurred. And in the tumors with relatively wide negative margins (≥ 2 mm), 1.1 percent recurred (Rosenberger LH, et al. J Clin Oncol. 2020;39[3]:178–189). The study’s authors concluded that recurrence is not associated with wider negative margins, final margin status (positive versus negative), surgery type, mitosis, tumor border, or age. “So it seems from the current studies that wide margins are not needed for benign phyllodes tumors,” Dr. Li said, “and treatment should be personalized.” (The study also reports the local recurrence rates of borderline and malignant phyllodes tumor.)
In practice, Dr. Li said, “we often face the situation of differentiating metaplastic carcinomas from malignant phyllodes tumors.” A DCIS or malignant carcinoma adjacent to a spindle cell lesion favors a metaplastic carcinoma. Cytokeratin, p63, and CD34 staining are helpful in differentiating the two. Metaplastic carcinomas typically are positive or focally positive for cytokeratin and p63 and negative for CD34. But some malignant phyllodes tumors can be focally positive for cytokeratin staining and p63, and some can be negative for CD34, he said. “So if CD34 is negative, it’s not that helpful. But if CD34 is positive, you can virtually rule out metaplastic carcinoma.”
Charna Albert is CAP TODAY associate contributing editor.