
April 2015—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. Here, this month, is case No. 7. (See the February, August, and September 2013 and the May, June, and November 2014 issues for the first six.) AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, treatment, and more. Case report No. 7 comes from the University of New Mexico School of Medicine. If you would like to submit a case report, please send email to the AMP at amp@amp.org. For more information about the AMP, visit www.amp.org.
Daniel Bustamante, MD
Devon Chabot-Richards, MD
A 46-year-old male presented to the emergency room with recent history of exercise-induced dyspnea and presyncope. He also states that for the past two to three months he has had occasional melanotic stools along with a recent 10-pound weight loss due to lack of appetite. CBC showed a hemoglobin/hematocrit of 5.9/19. The patient had a colonoscopy the next day that revealed a large 6 cm mass within the cecum. The mass was unresectable due to the large size but biopsies were obtained. Histology returned a few days later showed colonic mucosa with high-grade dysplasia with a focus very suspicious for invasion. CT preliminary scans during the hospital admission showed the mass near the ileocecal valve with possible involvement of the appendix and associated with an increased number of mesenteric lymph nodes in that region.
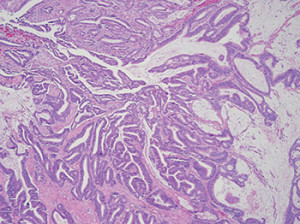
Fig. 1: Example of colorectal adenocarcinoma with mucinous features.
He soon underwent a right hemicolectomy with no complications. Histology of the hemicolectomy specimen showed colorectal adenocarcinoma, with moderately differentiated histology with multifocal mucinous pools containing signet ring cells (Figs. 1 and 2). The presence of signet ring cells suggests a more aggressive tumor. Thirty-two lymph nodes were identified in the specimen and were negative for malignancy. Pathologic stage was pT2 pN0.
Given the right colon location, mucinous differentiation, and the patient’s young age, an inherited loss of mismatch repair gene function, such as Lynch syndrome, was suspected. Lynch syndrome is a familial cancer syndrome associated with increased risk of colorectal and endometrial cancers, as well as other less common tumors. Lynch syndrome is most commonly caused by germline mutations in mismatch repair (MMR) genes MLH1 and MSH2. Other MMR genes such as MSH6, PMS1, and PMS2 are only rarely affected. To further investigate and identify possible inherited colorectal carcinoma, microsatellite testing was performed.
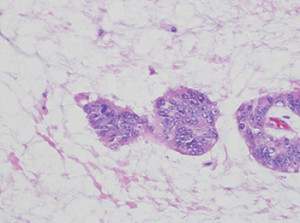
Fig. 2: High-power view of malignant cells in pools of mucin.
Microsatellites are short, tandem repeated DNA sequences from one to six base pairs in length that are susceptible to incorrect sequence placement or mismatch during DNA replication. These areas are normally monitored and constantly fixed by MMR proteins. When MMR genes and proteins are mutated or non-functioning, microsatellite instability, a change in length of a microsatellite allele due to insertion or deletion of repeating units, results. The National Cancer Institute has identified five markers that should be screened to identify instability. If greater than two of these are unstable, the sample is defined as microsatellite instability-high (MSI-H). MMR gene function can be impaired sporadically via epigenetic methylation or through inherited mutation, as in Lynch syndrome. Thus, if microsatellite instability is high, further testing is indicated to distinguish between sporadic MMR gene silencing through promoter methylation and an inherited mutation. Screening can also be done using immunohistochemical stains for the MMR proteins MLH1, MSH2, MSH6, and PMS2. If all four are positive, the possibility of Lynch syndrome is low. Absent staining of one or more is consistent with sporadic or hereditary loss of mismatch repair function.1,2
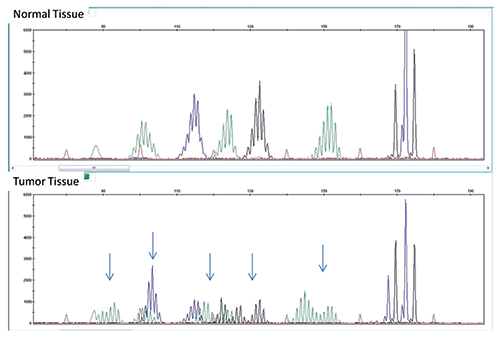
Fig. 3: MSI assay showing multiple sites of instability in tumor tissue (bottom) compared with normal tissue (top). A panel consisting of five mono/dinucleotide markers was used for MSI determination via multiplex PCR. The amplified products were separated by capillary gel electrophoresis. Comparison of peak patterns with a shift in PCR product size of the tumor when compared with normal represents instability. The arrows represent shifts in base pairs as compared with normal tissue.
Our patient’s tumor showed instability in multiple microsatellite loci, consistent with MSI-H (Fig. 3). At our institution our reflex test of choice for an MSI-H result is an MLH1 methylation assay. MLH1 is the most commonly mutated mismatch repair gene in Lynch syndrome. If part of an inherited process, an individual often inherits one mutated copy and later on in life develops a mutation in the second copy. More commonly, though, the MLH1 gene is silenced through acquired promoter methylation. The reflex MLH1 assay tests for the presence of methylated nucleotides by PCR of bisulfite-treated DNA. This assay tests for global methylation and cannot distinguish between cases with methylation of one copy and cases with methylation of both copies. Patients with normal promoter methylation are presumed to have Lynch syndrome and MMR gene sequencing may be performed. BRAF mutation testing is an alternative with similar efficacy that can also be used to screen for Lynch syndrome, as BRAF mutations are not seen in patients with Lynch syndrome but are often positive in sporadic (methylated) colorectal cancers. Patients who are negative for BRAF mutation may then be sent for MMR gene sequencing.
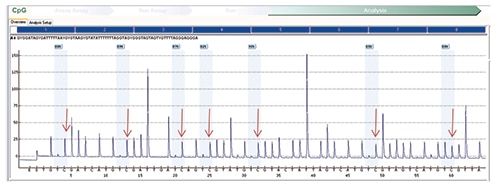
Fig. 4: MLH1 methylation assay on tumor showing an increased percentage of cytosine nucleotides in sequence, a surrogate for methylated nucleotides after bisulfite treatment. (Red arrows indicate an increased percentage of cytosine nucleotides, a marker of methylated nucleotides.)
Our patient’s assay results showed an increase in methylation of the MLH1 promoter region detected in both tumor and normal tissue (Fig. 4 and Fig. 5, respectively). This pattern of methylation is unusual because typically the normal tissue is used as a control and is often negative for methylation. That both the tumor and normal tissue are methylated raises the possibility of a rare constitutional (germline) epigenetic abnormality. To further investigate this possibility, the degree of MLH1 methylation on the patient’s peripheral blood was tested. If the patient harbors a germline (constitutional) methylation abnormality, his blood cells should also share the same molecular findings. The percentage of methylation in our patient’s peripheral blood specimen (Fig. 6) was comparable to the percentage previously detected on the DNA extracted from the uninvolved/control tissue (69 percent versus 58 percent). Compared with the overall hypermethylation percentage of the actual colorectal tumor, 87 percent, the lower methylation percentage in normal tissue and peripheral blood suggests the possibility of homozygous hypermethylation of MLH1 in the patient’s tumor (87 percent) and inherited heterozygous MLH1 methylation in normal tissue and in DNA obtained from peripheral blood. It is possible that MLH1 mutations were present at the genetic level, though no further testing was pursued on this patient’s sample and gene sequencing was not performed. In a patient with germline methylation of one copy of MLH1, it is possible that loss of the second copy through epigenetic silencing could cause complete loss of mismatch repair function.
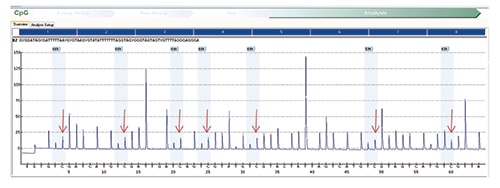
Fig. 5: MLH1 methylation assay on normal (control) tissue shows a similar increase in the percentage of cytosine nucleotides in sequence consistent with the presence of methylated nucleotides on normal tissue.
The finding of a suggested germline epimutation has been described in publications, though it appears to be rare with still undefined medical consequences and cancer risks.3,4 As opposed to Lynch syndrome, which is inherited as an autosomal dominant disease, and where children are at 50 percent risk of having inherited the mutation, germline hypermethylation appears to be a transient phenomenon that is rarely transmitted to the subsequent generation. It is thought that individuals with germline hypermethylation are possibly susceptible to the same cancer risks observed in individuals with Lynch syndrome due to germline mutation in the MLH1 gene. Given this assumption, until further discovery of the effects of germline hypermethylation or cancer risks are established, we decided it would be safest to screen our patient according to the recommendations made for individuals with Lynch syndrome. This includes annual colonoscopy and biannual upper endoscopy, as well as urine cytology and physical exam with review of systems every year.
- Raedle J, et al. Bethesda guidelines: relation to microsatellite instability and MLH1 promoter methylation in patients with colorectal cancer. Ann Intern Med. 2001;135(8 Pt 1):566–576.
- Pérez-Carbonell L, et al. Methylation analysis of MLH1 improves the selection of patients for genetic testing in Lynch syndrome. J Mol Diagn. 2010;12(4):498–504.
- Hitchins MP, Ward RL. Constitutional (germline) MLH1 epimutation as an aetiological mechanism for hereditary non-polyposis colorectal cancer. J Med Genet. 2009;46(12):793–802.
- Pineda M, et al. MLH1 methylation screening is effective in identifying epimutation carriers. Eur J Hum Genet. 2012;20(12):1256–1264.
Test yourself
Here are three questions taken from the case report. Answers are online now at www.amp.org/casereviews and will be published next month in CAP TODAY.
1. Which is considered a feature of an MSI-H colon cancer?
a) Many tumor infiltrating lymphocytes
b) Mucinous features and signet ring cells
c) Right side location
d) None of the above
e) All of the above
2. If a patient is found to have Lynch syndrome, what are the chances that his or her grandchild will inherit the mutation?
a) 50 percent
b) 0 percent
c) 25 percent
d) 100 percent
e) None of the above
3. Which relationship between microsatellite repeats and mismatch repair proteins is correct?
a) Mismatch repair proteins methylate microsatellite repeats.
b) Mismatch repair proteins correct microsatellite sequences, which often have a high rate of error.
c) Mismatch repair mutations are due to microsatellite sequence methylation.
d) Microsatellite instability is most often due to inherited mutations in the mismatch repair proteins.
[hr]
Dr. Bustamante is a pathology resident and Dr. Chabot-Richards is an assistant professor in the Department of Pathology, University of New Mexico School of Medicine, Albuquerque.
![]()