
Susan D. Rollins, MD
Donna K. Russell, CT(ASCP)
August 2017—Adequate and high-quality cell block preparations can be a useful adjunct to cytologic smear preparations and touch imprint cytology. Adequate cell blocks allow for additional studies and can provide a specific diagnosis and information essential for targeted treatment plans. Cell blocks can be prepared from most cytology specimens such as fine needle aspirations, body cavity fluids, washings, brushings, and gynecologic and nongynecologic liquid-based specimens.
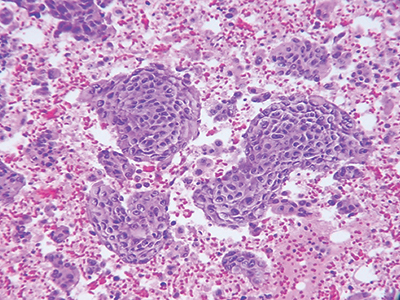
Fig. 1. Plasma thrombin cell block preparation, hematoxylin and eosin stain, 20×.
Rapid on-site evaluation of fine needle aspiration material can assist with optimal specimen collection and triage of material. Collaboration with the patient-centered team at the time of immediate assessment can contribute to protocols and improve patient outcomes. Dedicated fine needle aspiration passes for cell block material facilitate exceptional cell block preparation. Relying solely on rinses from fine needle aspiration specimens may result in suboptimal cell block preparations. Obtaining ample cell block material allows for additional ancillary studies like immunocytochemical stains, fluorescence in situ hybridization, and other molecular studies to be performed on the cytology specimen. These studies often aid in definitive classification of the lesion and identify treatment options. FNA with cell block is not only less invasive but also cost-effective when compared with the additional expenses of procuring a surgical biopsy specimen. Cell blocks can be archived for future diagnostic and research purposes while allowing the original smears to be preserved. An added benefit is that emerging microwave processing techniques can be used for small biopsies and cell blocks and can expedite turnaround time in rush cases.
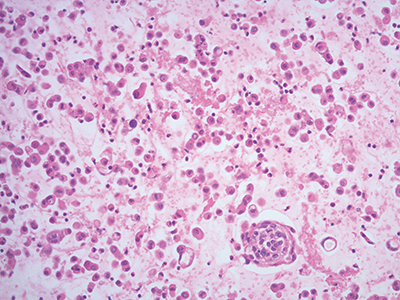
Fig 2. Collodion bag cell block procedure, hematoxylin and eosin stain, 20×.
Different cell block preparation methods use different mediums to rinse, transport, and fix specimens. Saline and Roswell Park Memorial Institute (RPMI) medium allow flexibility for future ancillary studies such as flow cytometry. Liquid-based preparations such as those used with Pap and urine specimens contain alcohol (methanol or ethanol). Cell block specimens may also come in contact with alcohol before the cell block preparation step if cells are removed from an ethanol-fixed direct smear.
Neutral buffered formalin is considered the universal fixative. Other fixatives include a formalin/95 percent ethanol rinse and Nathan alcohol formalin substitute (nine parts 100 percent ethanol/one part 40 percent formaldehyde), which is considered a less toxic fixative. Methanol is used as a fixative for the automated Cellient cell block technique. Many ancillary testing platforms use and have been validated for formalin-fixed, paraffin-embedded tissue. False-negative immunocytochemical stain results may occur with non-formalin fixation methods due to altered antigenicity. Laboratories need to validate alcohol-fixed and formalin-fixed cell blocks for immunocytochemical and molecular techniques. Varghese, et al., performed a validation study, published in Modern Pathology, that compared immunocytochemical stain results obtained from cell block material with immunohistochemical results from matched surgical specimen blocks. Forty-one specimens were used, with 118 stains performed with adequate controls. The cell block staining pattern matched that of surgical cell blocks on 113 stains, with a concordance rate of 95.7 percent.
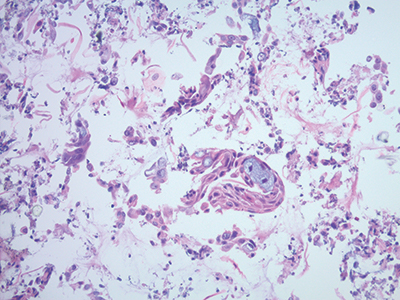
Fig. 3. Collodion bag cell block procedure, hematoxylin and eosin stain, 20×.
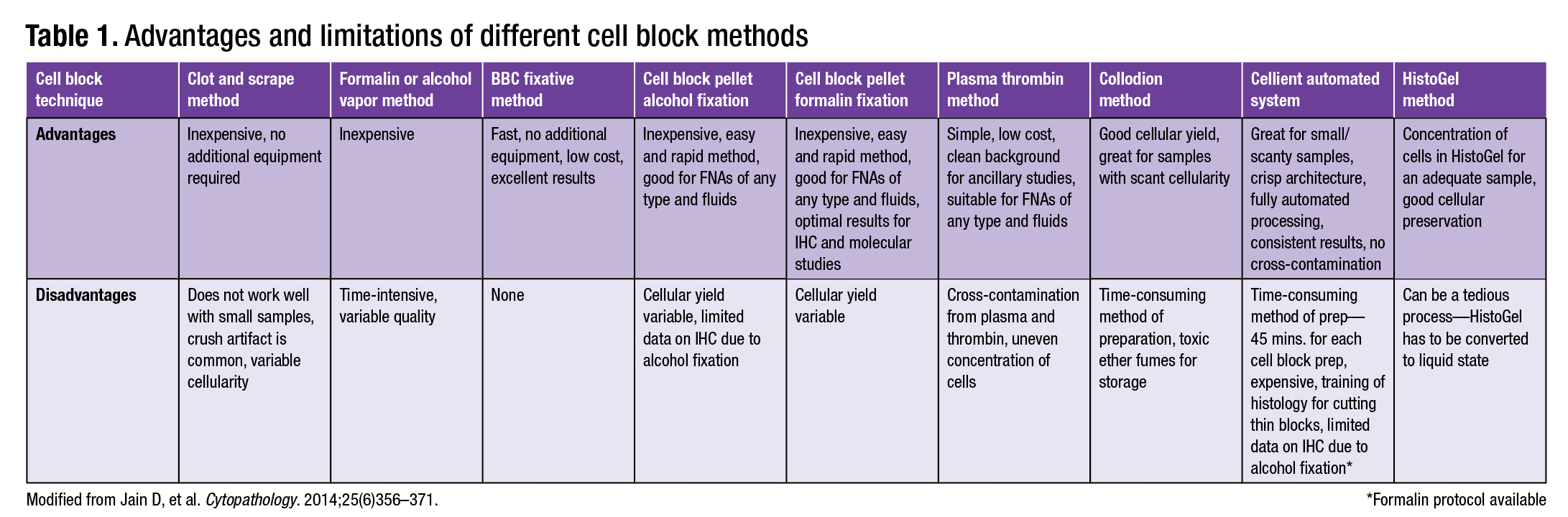
Laboratories use different approaches to cell block preparation, ranging from the low-tech “poor man’s vapor fixed” method to the high-tech Cellient automated cell block system. Several cell block methods are described here, and the advantages and limitations of different preparations are presented in Table 1 (page 68; modified from Jain, et al.). Laboratory resources, specimen volume, types of specimens prepared, and workflow must be considered when a laboratory is determining which cell block preparation method to employ.
The various cell block techniques follow. All cassettes and conical centrifuge tubes should be labeled in accordance with the laboratory’s protocol.
Clot and scrape method. A cell block can be obtained by expressing the contents of the aspirated specimen onto a glass slide. This allows the specimen to dry/clot; the material can then be scraped off the slide and wrapped in tissue paper. The specimen is placed in a histology cassette and neutral buffered formalin is added before routine processing in the histopathology laboratory.
BBC cell block fixative method. Use a needle to rinse the specimen in 1 mL of BBC Cyto-CellBlock Fix in a non-coated tube. Invert the tube onto appropriate filter paper. After the fluid has drained, scrape tissue on the paper into a small area. The specimen is then processed routinely in the histopathology laboratory.
Plasma thrombin cell block preparation. This is the most popularly used cell block method. The cell block is prepared from the pellet of centrifuged cell suspension by adding plasma and thrombin to enmesh the cellular material in a clot (Fig. 1).
Add up to 20 mL of specimen to a falcon tube and centrifuge for 10 minutes at 1,650 rpm. After centrifugation, the supernatant is decanted. Add 0.5 mL of plasma and two drops of three percent aqueous eosin to the sediment and vortex briefly. Next, 0.25–0.5 mL of reconstituted thrombin is added and the solution is quickly agitated. A clot will form within 30–60 seconds. Place clot into a labeled cassette containing formalin. The specimen is then processed routinely in the histopathology laboratory.
Collodion bag cell block procedure. This method concentrates the cells for a more adequate cell block preparation in a collodion bag (Figs. 2 and 3).
Preparation of collodion bag tubes. Pour collodion reagent into 15-mL glass tubes and fill to top. Allow the collodion to sit in the tubes for 10–15 minutes. Pour the collodion back into the reagent bottle, rotating the tube while pouring. Place the tube upside down in the test tube rack and allow it to remain about 30 minutes until dry. When dry, fill the tubes with distilled water. If the bag is not sufficiently dry, the tube will appear opaque and the bag cannot be used and should be discarded. Discard distilled water just before use. The collodion bag can be stored for up to one week.
Preparation of cell block in collodion bag. Pipette specimen into collodion bag. Centrifuge an aliquot of fluid in the tube containing the collodion bag for 10 minutes at 2,500 rpm. Carefully pipette off supernatant. Using forceps, assemble the edge of the collodion bag on the lip of the tube. Lift the bag from the tube with forceps and tie with cotton string just above the pellet. Cut off excess collodion bag and string with scissors. Place remaining bag in a tissue cassette, and place the cassette in a specimen cup filled with 10 percent neutral buffered formalin. The specimen is then processed routinely in the histopathology laboratory.
Shandon Cytoblock method. This technique is a manual cell block preparation product. The product concentrates the cells by cytocentrifugation in a Thermo Shandon Cytospin using Cytoblock cassettes and reagents, which are available in a kit. The Cytoblock cassette is then placed in neutral buffered formalin. The specimen is then processed routinely in the histopathology laboratory.
This technique replaces time-consuming and costly agar and thrombin techniques and requires phosphate-free fixative on the histology tissue processor, such as Zinc Formal-Fixx, Formal-Fixx, or Glyo-Fixx (formalin substitute), or simply bypass formalin fixation steps on the processor. Cyto-block can also be run on a rapid biopsy program.
Cellient automated cell block system. This technique is commonly used when the specimen consists of small tissue fragments and is made from the liquid-based preparation vial. It is the first fully automated cell block system (Fig. 4).
The specimen is centrifuged in a 60-mL vial for five minutes at 2,400 rpm. Once the specimen is spun down, the supernatant is poured off. Only three drops of cell sediment are added to PreservCyt vial to be fixed. The vial containing the sample is placed into the Cellient automated cell block system for processing. Average prep time is 45 minutes per cell block. The specimen is then processed routinely in the histopathology laboratory.
HistoGel method. This technique is used for cytology specimens with a predominance of individually scattered cells. The protocol involves steps to concentrate the cells of interest along the plane parallel to the cutting surface of the cell block. This technique can be used for cell block preparations of cervical ThinPreps, which are usually less cellular and may contain only scattered individual abnormal cells.
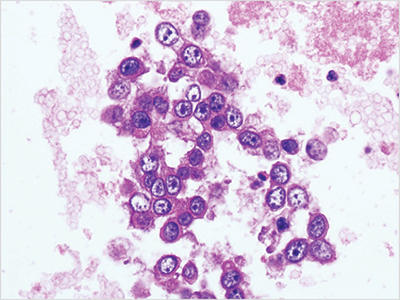
Fig. 4. Cellient automated cell block system preparation (ascitic fluid preparation, adenocarcinoma, hematoxylin and eosin stain, 40×).
The specimen is centrifuged at 3,000 rpm for five minutes. The supernatant is poured off and the sediment remains in the tube. HistoGel (HG) is liquefied by melting it on medium power in the microwave for five to 15 seconds. Enough HG to cover the sediment (about 0.5 mL) is added to the tube and mixed with the sediment. Allow HG to solidify (two to three minutes at room temperature or quicker if using a cooling block). The solidified HG sediment is dislodged from the bottom of the tube by squirting 10 percent neutral buffered formalin into the side of the tube. Remove cell block sediment and place in filter paper in a labeled cassette in formalin. The specimen is then processed routinely in the histopathology laboratory.
Care should be taken when facing and cutting the paraffin block because the tissue fragments may be right at the surface of the block.
Tissue coagulum clot method. This method allows a clot to form in the lumen of the fine needle aspiration tip. The clot is then transferred directly to formalin for fixation. The technique prevents the loss of diagnostic material.
To prepare cell blocks using the tissue coagulum clot method, the material within the 21-gauge/22-gauge cytology needle is gently pushed out using the wire stylet found with the needle kit, instead of using a syringe to expel the material into saline for cell block preparation. As the material exits the needle tip, it is collected onto a precut piece of filter paper with the needle tip directed in a tight circular motion to build up a cone-shaped coagulum of tissue and blood mixture. The clot is slightly air-dried on the filter paper, mostly to ensure the coagulum is solid and the cellular elements are not dispersed in a liquid medium. The specimen is wrapped in tissue paper and placed in a cassette and container of formalin. The specimen is then processed routinely in the histopathology laboratory.
Formalin or alcohol vapor method. A specimen is fixed using formalin or alcohol fumes. This method requires no additional equipment and minimal reagents. The material is expelled from a fine needle aspiration to form a small clot on the inside of a lid of a universal specimen container. The lid is inverted and a ball of tissue paper is pushed into the bottom of the container. About 2 mL of formalin is added to the container and soaks into the tissue paper.
If alcohol is used, two isopropyl alcohol phlebotomy swabs are placed in the bottom of the container.
The container is left in the inverted position for at least six hours at room temperature. By this time the specimen has been fixed by formalin or alcohol vapor and has become solid. It is taken off the lid by adding formalin to break the suction between specimen and lid. The specimen should be placed in a cassette and formalin added as soon as it is removed from the lid. The specimen is then processed routinely in the histopathology laboratory.
Cell block technique for cell pellet or tissue fragments—method No. 1 (using 95 percent alcohol). This commonly used method uses alcohol for centrifuging the specimen that is submitted in a preservative alcohol-based medium.
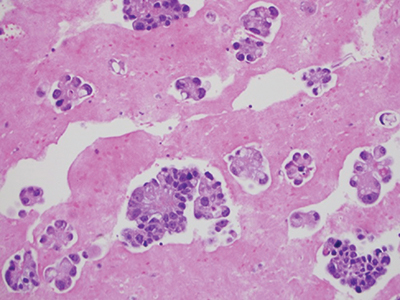
Fig. 5. Cell block technique for cell pellet or tissue fragments—method No. 2 using saline, RPMI, or formalin for collection, hematoxylin and eosin stain, 20×.
Label a 50-mL conical centrifuge tube with multiple patient identifiers. Decant up to 50 mL of specimen into the labeled tube. Store an aliquot (if necessary) for ancillary testing. Centrifuge specimen with fixative (95 percent alcohol) or preservative medium (e.g. CytoLyt) for 10 minutes (2,500 rpm). Decant the supernatant and thoroughly vortex the specimen (determine if you need a second centrifugation step to lyse grossly bloody specimens or hardened/compact cell pellet). Add aliquot of 95 percent alcohol to specimen, mix thoroughly, centrifuge again. Decant alcohol completely.
At this point, based on the size of the cell pellet, you can choose the most appropriate method. If the cell block pellet is very small and/or sediment is scant or does not pack well, use an alternative technique such as HistoGel or agar.
Remove cell pellet from the conical tube with a spatula; section as necessary to match the width of a nickel. Place in a tissue cassette. Place a sponge with a hole cut out of the center in the bottom of the labeled tissue cassette. Sponges may not be necessary with a large, well-solidified cell pellet.
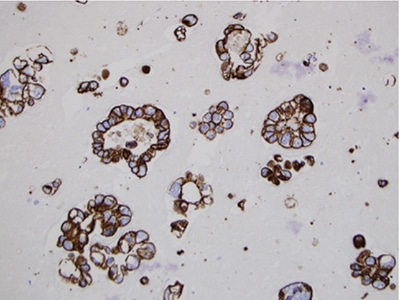
Fig. 6. Cell block technique for cell pellet or tissue fragments—method No. 2, MOC 31 immunocytochemical stain, 20×.
Place an appropriate size piece of lens paper on top of the blue sponge. Place the cell block section on top of the lens paper, centered in cassette. Place a second piece of lens paper, at a 45-degree angle, on top of the cell block section. Place a second sponge with a hole cut out of the center on top of the second piece of lens paper. Securely snap the cassette cover in place and place the cassette in formalin. The specimen is then processed routinely in the histopathology laboratory.
Cell block technique for cell pellet or tissue fragments—method No. 2 (using saline, RPMI, or formalin for collection). This method is commonly used for cell block preparation (Figs. 5 and 6).
Transfer material to a 50-mL conical centrifuge tube and label with multiple patient identifiers. Set the centrifuge to run at 2,400 rpm for five minutes to achieve a concentrated cell button. Pour off supernatant. The button may not be cohesive and it may start to fall apart as the supernatant is being removed. Should this happen, equal parts neutral buffered formalin can be added to help fix the specimen. This mixture should sit for 30–60 minutes to allow material to set. The hardened cell button is then removed with a metal spatula by loosening the edges until it breaks free of tube. The button is then placed on histology tissue paper that has been moistened with neutral buffered formalin. Tissue paper is folded up around the button and placed in a histology cassette.
 Once all steps are complete, the cassette and its contents are put in a specimen cup filled with buffered formalin, which should cover the cassette entirely. Allow the specimen to fix for at least 30 minutes or longer. The specimen is then processed routinely in the histopathology laboratory.
Once all steps are complete, the cassette and its contents are put in a specimen cup filled with buffered formalin, which should cover the cassette entirely. Allow the specimen to fix for at least 30 minutes or longer. The specimen is then processed routinely in the histopathology laboratory.
If the cell block pellet is very small and/or sediment is scant or does not pack well, use an alternative technique such as HistoGel or agar. Sponges may not be necessary with a large, well-solidified cell pellet.
- Castro-Villabon D, Avello Y, Ruiz N, Rodriguez-Urrego PA. Implementation of routine thromboplastin-plasma cell block technique in the evaluation of non-gynecologic specimens. J Microscopy Ultrastructure. 2014;2:177–181.
- Gill GW. Cell block preparation. In: Cytopreparation: Principles and Practice, Essentials in Cytopathology. New York, NY: Springer-Verlag; 2013.
- Jain D, Mathur SR, Iyer VK. Cell blocks in cytopathology: a review of preparative methods, utility in diagnosis and role in ancillary studies. Cytopathology. 2014;25(6):356–371.
- Khan S, Omar T, Michelow P. Effectiveness of the cell block technique in diagnostic cytopathology. J Cytol. 2012;
29(3):177–182. - Kulkarni MB, Desai SB, Ajit D, Chinoy RF. Utility of the thromboplastin-plasma cell-block technique for fine-needle aspiration and serous effusions. Diagn Cytopathol. 2009;37(2):86–90.
- Mayall F, Chang B, Darlington A. A review of 50 consecutive cytology cell block preparations in a large general hospital. J Clin Pathol. 1997;50(12):985–990.
- Mayall F, Darlington A. The poor man’s cell block. J Clin Pathol. 2010;63(9):837–838.
- Nathan NA, Narayan E, Smith MM, Horn MJ. Cell block cytology. Improved preparation and its efficacy in diagnostic cytology. Am J Clin Pathol. 2000;114(4):599–606.
- Varsegi GM, Shidham V. Cell block preparation from cytology specimen with predominance of individually scattered cells. J Vis Exp. 2009;29:e1316.
- Varghese S, Martin A, Russell D, McMahon L, Fiscella J. Comparison of immunohistochemistry staining performed on cell blocks versus surgical blocks from the same tumor. Mod Pathol. 2017;30(S2):520A–521A.
- Wagner DG, Russell DK, Benson JM, Schneider AE, Hoda RS, Bonfiglio TA. Cellient automated cell block versus traditional cell block preparation: a comparison of morphologic features and immunohistochemical staining. Diagn Cytopathol. 2011;39(10):730–736.
- Yung RC, Otell S, Illei P, et al. Improvement of cellularity on cell block preparations using the so-called tissue coagulum clot method during endobronchial ultrasound-guided transbronchial fine-needle aspiration. Cancer Cytopathol. 2012;120(3):185–195.
- Zanchelli-Astran G, Wood M, Bibbo M. Making the diagnosis with only two levels of nongynecologic cell blocks as opposed to three is more cost effective. Diagn Cytopathol. 2010;38(5):374–376.
[hr]
Dr. Rollins is director of the Outpatient Cytopathology Center, Johnson City, Tenn. Donna Russell is in the Department of Pathology and Laboratory Medicine, University of Rochester Medical Center, Rochester, NY. Both are members of the CAP Cytopathology Committee.