
Stacey Barron Miller, MD
Chengquan Zhao, MD
August 2016—The Bethesda System for Reporting Cervical/Vaginal Cytologic Diagnoses was developed to establish standardized terminology among pathologists for communicating to clinicians the findings of a Pap test.1 The Bethesda System has also facilitated the examination of the epidemiology and pathogenesis of cervical disease, with a focus on low-grade and high-grade squamous intraepithelial lesions (LSIL and HSIL, respectively) and their relationships to human papillomavirus infection and progression to invasive cervical carcinoma. This accumulating knowledge has allowed for the development of cervical cancer screening algorithms and management guidelines set forth by the American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology. These algorithms and guidelines are subject to modification as knowledge of cervical disease advances and data on risk of disease accumulate. It is universally accepted at this time that women with cytologic findings of HSIL require colposcopy or surgical excision given the high risk of identifying a CIN2+ lesion on histologic examination. The clinical management of LSIL, however, has continued to evolve with the changing screening recommendations, namely HPV co-testing and longer screening intervals.
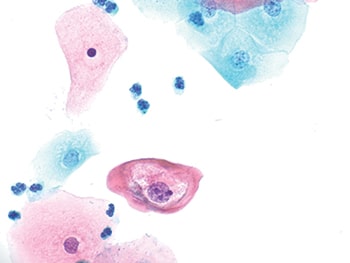
Fig. 1. One typical koilocyte (600×) with peri-nuclear cavitation and nuclear enlargement.
LSIL is a category recognized in the Bethesda System that is characterized by the cytologic features of HPV infection. Classically, LSIL is a lesion of intermediate and superficial squamous cells with enlarged, hyperchromatic nuclei with irregular nuclear contours and perinuclear cytoplasmic clearing, referred to as koilocytic change.1 Figs. 1–4 demonstrate types of LSIL in Pap tests. LSIL is reported in 1.3 to 2.5 percent of all Pap tests, with the highest reporting rates in liquid-based Pap tests.2 It has been demonstrated that 55 to 89 percent of women with LSIL Pap tests are positive for high-risk HPV (hr-HPV),3,4 with the Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) documenting 83 percent of LSIL cases as hrHPV positive.5 With the initiation of routine hrHPV co-testing, hrHPV-positive rates with LSILs have been shown to be significantly higher in women under age 30 (88.8 percent) compared with older populations (80.1 percent at 30–39 years and 77.2 percent at ≥60 years).6 Additionally, the histopathologic detection rate of CIN2+ lesions in LSIL patients is 14.5 percent and 3.7 percent in hrHPV-positive and hrHPV-negative women, respectively.6
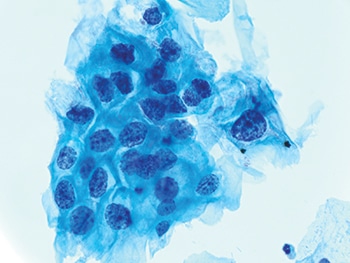
Fig. 2. One cluster of LSIL cells (600×) with nuclear enlargement and hyperchromasia. HPV-related cytoplasmic changes are not required for LSIL.
HSIL remains a distinct category from LSIL because it is more often associated with a higher rate of hrHPV positivity (reported at 95.7 percent), viral persistence, and a higher risk of progression to invasive cervical carcinoma.7 At colposcopy, CIN2+ lesions are found in approximately 50 to 70 percent of women with HSIL, while invasive cervical cancer is found in about two percent.8 The overall five-year risk of invasive cervical cancer approaches eight percent in women age 30 and older after a diagnosis of hrHPV-positive HSIL.9 For women with the rare finding of a hrHPV-negative HSIL Pap test result, the risk of invasive cervical carcinoma remains elevated at seven percent.9 The recommendation of immediate biopsy or surgical resection in these women has remained unchanged since the first publication of the management guidelines.
In recent years, the cytologic interpretation of “low-grade squamous intraepithelial lesion, cannot exclude high-grade squamous intraepithelial lesion” (LSIL-H) has become more often used by pathologists when “atypical squamous cells, cannot exclude HSIL” (ASC-H) are encountered in a background of LSIL. These findings represent a small yet distinct group of women with a hrHPV-positive rate of 90.5 percent, which is significantly higher than that of LSIL or ASC-H (80.2 percent and 54.3 percent, respectively) and slightly lower than HSIL (95.7 percent).7 More important, the histopathologic detection rate of CIN2+ lesions in LSIL-H (29.4 percent) falls intermediate between HSIL (70.5 percent) and LSIL and ASC-H (12.9 percent and 17.2 percent, respectively).7 LSIL-H, however, has not been clearly recognized as a distinct category in the Bethesda System, and, as a result, no corresponding recommendations or management guidelines have been established for women with this Pap test result. Management at this time is based on clinical judgment with recommendations from the current guidelines.
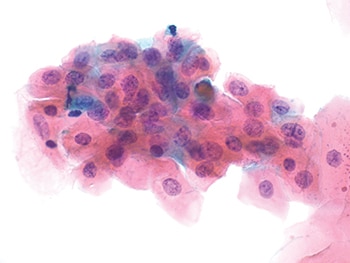
Fig. 3. One cluster of LSIL cells (600×) with nuclear enlargement and eosinophilic cytoplasm and no perinuclear cavitation.
In 2001, the initial ASCCP and ACOG consensus guidelines recommended immediate colposcopy for all non-adolescent premenopausal women with LSIL, regardless of HPV results. These recommendations were facilitated by the consensus that there was little utility for routine reflex HPV testing in managing LSIL Pap test results, given the high percentage of LSIL cases found to be hrHPV-positive. However, subsequent publication of the ALTS data confirmed that five to 16 percent of women with LSIL Pap results tested hrHPV-negative.10 Recent data have further demonstrated that the risk for histopathologic CIN2+ and CIN3+ for women with hrHPV-negative LSIL results may be as low as three percent and 0.2 percent, respectively, significantly less than the risk for patients with hrHPV-positive LSIL and similar to the risk for patients with ASC-US alone.11
Several explanations for the presence of hrHPV-negative LSIL have been put forward, including non-oncogenic HPV infections, cut-point–related sensitivity limitations implemented to enhance specificity of hrHPV tests, false-positive Pap test results, and viral clearance before the time of histologic sampling.6,10 These findings have led to the concept that women with hrHPV-negative LSIL co-testing results might be better managed less aggressively. As a result, the most recent cervical screening test management guidelines, released in 2012, recommend that the preferred follow-up method for women with hrHPV-negative LSILs is to repeat cytology and hrHPV co-testing at one year, deferring immediate elective colposcopy.12
Women with hrHPV-negative LSIL are not the only subgroup of LSIL with an alternative management strategy in which immediate colposcopy may be deferred. Young women under age 25 have a low risk of progression to cervical cancer owing to their high clearance rate of HPV infection and high regression rates of cervical disease. Therefore, young women with LSIL, both pregnant and nonpregnant, are initially managed with repeat cytology at 12 months. Colposcopy is deferred unless ASC-H+ or ASC-US+ is detected at 12 and 24 months follow-up, respectively. The preferred management of non-adolescent pregnant women is colposcopy; however, it is acceptable to defer colposcopic examination until at least six weeks postpartum due to the high rate of postpartum regression of cervical disease and the low rate of cervical cancer. Similarly, postmenopausal women with LSIL are managed optimally with hrHPV testing, repeat cytologic testing at six months and 12 months, followed by colposcopy if ASC-US+ is detected or if they test hrHPV-positive.
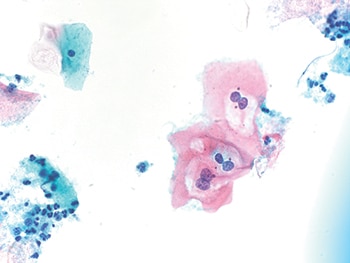
Fig. 4. One small cluster of LSIL cells (600×) with perinuclear cavitation, binucleation, and slightly enlarged nuclei.
The enhancement of cervical screening with hrHPV co-testing and installation of longer screening intervals has made it possible to accumulate new data on LSIL including age-based risk stratification of disease progression with regard to hrHPV infection. These data have supplemented the initial findings of the ALTS trial, leading to modification of management guidelines. In addition, new cytologic categories, such as LSIL-H, have been identified that impart increased risk of cervical disease to patients but lack clinical management guidance. As the examination of the relationship between hrHPV and cervical disease continues and new data accumulate, there may be a shift from the dependence on a broad consensus guideline to a more personalized approach to management.
- Solomon D, Davey D, Kurman R, et al; Forum Group Members; Bethesda 2001 Workshop. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287(16):2114–2119.
- Eversole GM, Moriarty AT, Schwartz MR, et al. Practices of participants in the College of American Pathologists interlaboratory comparison program in cervicovaginal cytology, 2006. Arch Pathol Lab Med. 2010;134(3):331–335.
- Arbyn M, Martin-Hirsch P, Buntinx F, Van Ranst M, Paraskevaidis E, Dillner J. Triage of women with equivocal or low-grade cervical cytology results: a meta-analysis of the HPV test positivity rate. J Cell Mol Med. 2009;13(4):648–659.
- Arbyn M, Sasieni P, Meijer CJ, et al. Chapter 9: Clinical applications of HPV testing: a summary of meta-analyses. Vaccine. 2006;24(suppl 3):S78–S89.
- The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst. 2000;92(5):397–402.
- Heider A, Austin RM, Zhao C. HPV test results stratify risk for histopathologic follow-up findings of high-grade cervical intra-epithelial neoplasia in women with low-grade squamous intra-epithelial lesion Pap results. Acta Cytol. 2011;55(1):48–53.
- Barron S, Li Z, Austin RM, Zhao C. Low-grade squamous intraepithelial lesion/cannot exclude high-grade squamous intraepithelial lesion (LSIL-H) is a unique category of cytologic abnormality associated with distinctive HPV and histopathologic CIN 2+ detection rates. Am J Clin Pathol. 2014;141(2):239–246.
- Dunn TS, Burke M, Shwayder J. A ‘‘see and treat’’ management for high-grade squamous intraepithelial lesion Pap smears. J Low Genit Tract Dis. 2003;7(2):104–106.
- Katki HA, Schiffman M, Castle PE, et al. Five-year risks of CIN 3+ and cervical cancer among women with HPV-positive and HPV-negative high-grade Pap results. J Low Genit Tract Dis. 2013;17(5 suppl 1):S50–S55.
- Zuna RE, Wang SS, Rosenthal DL, et al. Determinants of human papillomavirus-negative, low-grade squamous intraepithelial lesions in the atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesions triage study (ALTS). Cancer Cytopathol. 2005;105(5):253–262.
- Barron S, Austin RM, Li Z, Zhao C. Follow-up outcomes in a large cohort of patients with HPV-negative LSIL cervical screening test results. Am J Clin Pathol. 2015;143:(4)485–491.
- Massad LS, Einstein MH, Huh WK; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121(4):829–846.
[hr]
Dr. Barron Miller is an assistant professor of pathology, Department of Pathology, Allegheny General Hospital, Pittsburgh, and Dr. Zhao is a professor of pathology, co-director of cytopathology, and director of FNA Service, Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center. Dr. Zhao is a member of the CAP Cytopathology Committee.