Barbara A. Crothers, DO
Diane Davis Davey, MD
August 2020—If the past decade was directed toward aligning medicine with a personalized approach to therapy, this decade should further realize the implementation of health care decisions tailored to the patient. The updated 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors take a large step in that direction by relying on the input of personal data into a free online application that provides suggested management planning based on patient history and prior Pap/HPV test results.1 The rapid changes in primary cervical cancer screening algorithms in the past decade have necessitated a reexamination of the thresholds for therapy and surveillance in the screened population. Current screening guidelines allow for primary HPV screening, cotesting using a Pap test and HPV testing, and Pap testing alone, depending on a woman’s age. This has resulted in a web of possible tests at variable intervals that may confound clinical decisions about appropriate follow-up.
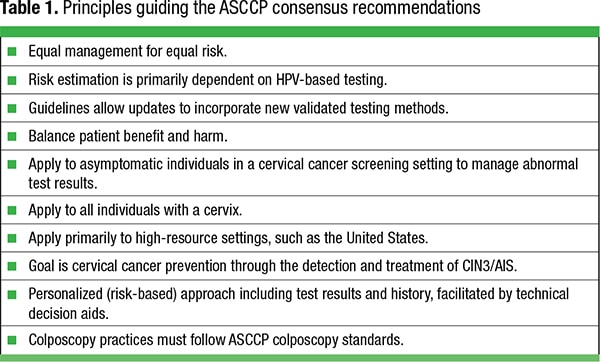
As with the guidelines of 2001, 2006, and 2012, the 2019 consensus guidelines were developed by a consortium of professional organizations, including the CAP, patient advocacy groups, and the federal government, and were sponsored by the American Society for Colposcopy and Cervical Pathology with data and scientific support from the National Cancer Institute. Decisions were based on literature review and supportive evidence to the greatest extent possible. Where evidence was lacking or weak, consensus opinion provided a starting point. The preponderance of evidence was derived from prospective longitudinal data from 1.5 million Kaiser Permanente Northern California beneficiaries followed for more than a decade.2 The guiding decision-making principle was “equal management for equal risk” in recognition of every woman having a unique set of circumstances and tests that define her likelihood of developing cervical cancer. Other guiding principles are presented in Table 1.
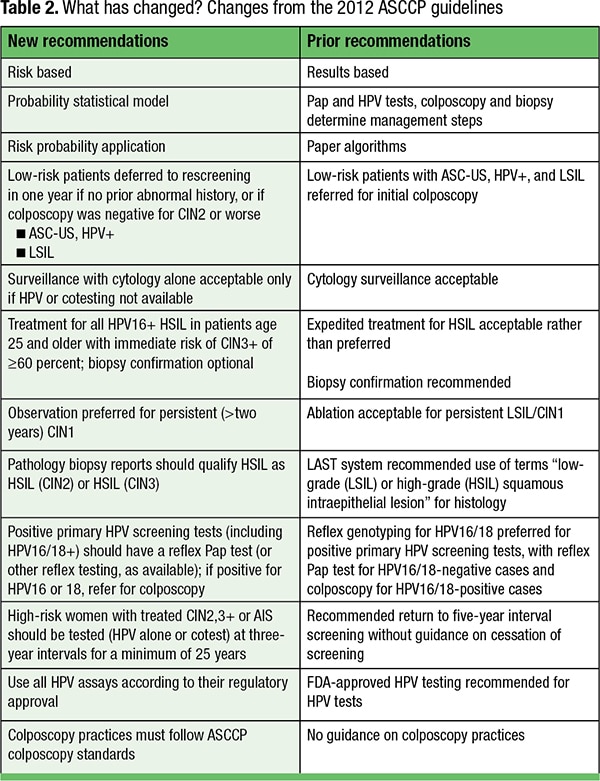
A major change is the shift from complex algorithmic management flowcharts to a probabilistic model of management that relies on the estimated risk that a woman will develop cervical cancer using a surrogate endpoint of five-year risk of cervical intraepithelial neoplasia (CIN) grade 3 (CIN3) or a more severe lesion (CIN3+, Table 2. This probability model incorporates a woman’s clinical history of prior CIN and screening or follow-up HPV and Pap tests to produce a probable risk value, with colposcopy, treatment, or more frequent surveillance recommendations for those at highest risk. The probability model allows women at lower risk to extend screening intervals and should deter unnecessary colposcopy, biopsies, and cervical excision.
The probability application is sponsored by the National Cancer Institute and will be available to providers free via the internet. Providers will be able to enter the available clinical, screening, and biopsy data on their patients into their smartphones to identify a patient’s estimated risk and link it to an appropriate management recommendation. Examples of clinical information that might modify risk include HPV subtypes, past cytology and HPV screening history, treated squamous intraepithelial lesion (SIL) or cancer, and hysterectomy. The application (decision aid) will be updated as data become available on the efficacy of new triage tests (such as p16/Ki-67 dual staining or new tumor biomarkers) or virulence of specific HPV subtypes.
Decades of research have confirmed the higher sensitivity of HPV testing (compared with a single Pap test) to detect cervical cancer precursors, defined as cervical intraepithelial neoplasia grade 2 (CIN2), CIN3, and adenocarcinoma in situ (AIS). HPV tests have higher negative predictive value, while Pap tests add high specificity and will play a greater role in defining women at intermediate risk and in deferring women at low risk to surveillance. For example, a woman with a low-grade squamous intraepithelial lesion (LSIL) Pap test who was previously HPV negative or has a concurrent HPV-negative result can be followed with surveillance only, rather than receive colposcopy and biopsy confirmation. In the current guidelines, the term “HPV-based testing” is used to incorporate both primary HPV tests (HPV-only primary screening test) and cotests (HPV testing coupled with a Pap test, with or without HPV genotyping). The probability models incorporated into the decision application are affected by HPV subtype and duration of infection, reflecting current understanding of the pathogenesis of cervical cancer.
The guidelines are intended for high-resource settings where HPV testing, Pap tests, and colposcopy are readily available, and they should be applied to asymptomatic individuals with a cervix subject to cervical cancer screening. Women who present with clinical symptoms, such as vaginal bleeding, require different management strategies. The primary goal of cervical cancer screening and management is to detect and eradicate precursor lesions (CIN2,3/AIS) to prevent cervical cancer while weighing these benefits with the risk of under- or overtreatment. The ASCCP has published consensus colposcopy standards3 that emphasize the need to take multiple (two to four) targeted cervical biopsies in high-risk patients to detect precursor lesions, while additional untargeted biopsies provide little additional benefit in discovering lesions.
The guidelines establish five major action thresholds for clinical management: routine screening, one-year surveillance, three-year surveillance, colposcopy, and treatment.
Routine screening. Routine screening guidelines are not addressed by these recommendations and are determined under a separate process, but the current management guidelines assume women have initial (first-time) screening test results. Women ages 25 to 65 are candidates for primary HPV test screening (without a Pap test) at intervals of every five years. Cotesting (HPV test with a Pap test) may also be done every five years, but a Pap test alone every three years is also acceptable. These guidelines apply only to women who do not have HIV infection, DES exposure, an immunocompromised state, or cervical cancer. Women without a cervix (e.g. status post-hysterectomy) do not require screening if they have never had precancer or cancer.4 Individuals with negative screening tests remain in the “routine screening” population. Screening data constitute one of the minimal data points required for the decision aid.
Surveillance. Patient surveillance involves more intensive follow-up than recommended screening intervals and is based on the patient’s risk of developing cervical cancer. Individuals with a higher risk of CIN3+ than the general population, based on screening results and history, are prompted to return earlier for an office visit and HPV testing prior to the usual three- or five-year screening interval. This population harbors a risk of CIN3+ between the colposcopy threshold and a five-year screening interval. After review of evidence and data, the Surveillance Working Group retained the prior guideline intervals of one, three, and five years because the data supported cancer risk that aligned well with these intervals. The recommended follow-up test for surveillance is HPV-based testing (primary HPV test or cotest).
One-year surveillance. Individuals whose calculated risk (≥ 0.55 percent) is less than that of someone deferred for immediate colposcopy but greater than the three-year surveillance threshold are retested in one year.1 Individuals with a calculated risk below the threshold for immediate colposcopy (four percent) but above the three-year surveillance threshold (≥ 0.55 percent) are also in this category. Most of the following situations will result in a one-year surveillance recommendation:
- Negative for intraepithelial lesion or malignancy (NILM)/HPV positive.
- Two HPV-positive, Pap-negative results with colposcopy < CIN2.
- Low-grade squamous intraepithelial lesion (LSIL)/HPV negative.
- Histologic LSIL/CIN1 following HPV-positive atypical squamous cells-undetermined significance (ASC-US) or LSIL.
- ASC-US/HPV positive following documented negative HPV test, cotest, or colposcopy.
- LSIL following documented negative HPV test, cotest, or colposcopy.
Three-year surveillance. Individuals whose calculated risk (≥ 0.15 percent but < 0.55 percent) approximates that of an individual in the general screening population with a single negative Pap test are deferred for testing at three years. Most individuals with a colposcopy history of CIN1/LSIL and a current negative HPV test or cotest will fall into this window, as will those with an ASC-US screening Pap test that is HPV negative.
Five-year surveillance. Individuals with a calculated risk (< 0.15 percent) that approximates that of an individual in the general screening population following a single negative primary HPV test or cotest are deferred for testing at five years.
Colposcopy. Individuals with a calculated immediate risk (≥ four percent) of having CIN3+/AIS or cancer are referred to immediate colposcopy. This risk threshold was established using data on women with a CIN3+ diagnosis after immediate referral to colposcopy and not based on the overall long-term risk. The upshot of this recommendation is that most prior recommendations for direct referral to colposcopy still generally apply. For example, individuals with HSIL and atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion (ASC-H) Pap tests are referred to colposcopy and should have targeted biopsies regardless of HPV results and prior history. Similarly, women with either ASC-US or LSIL/HPV+ cotests and previous unknown or HPV+ results should have colposcopy. Colposcopy is recommended for patients with either HPV18+ or HPV16+ results. Conversely, individuals with LSIL Pap tests or ASC-US/HPV+ cotests who would have been referred for colposcopy may now fall into one-year surveillance if they have had a prior documented negative HPV test, cotest, or colposcopy.
In pregnant women with CIN2, CIN3, or AIS, surveillance colposcopy every 12 to 24 weeks is preferred with treatment delayed until postpartum, and readers are referred to guidelines for details.
Treatment. For individuals age 25 and older with calculated immediate risk of CIN3+ ≥ 60 percent, an excisional procedure without prior colposcopy or biopsy confirmation is preferred, but colposcopy with biopsy is also acceptable. This risk is based on the person’s prior history and current screening results. For example, a 28-year-old woman with an HSIL Pap test that is HPV16+ would be scheduled for an excisional procedure. For those age 25 or older with a calculated risk between 25 and 59 percent, treatment without biopsy confirmation or following colposcopy and biopsies is acceptable. Examples are HSIL Pap test results, regardless of HPV status, and ASC-H/ HPV+ results.
Treatment is designed to eradicate precancerous tumor cells that have the potential to evolve into invasive cervical cancer. Progression rates of CIN3 to invasive squamous cell carcinoma cannot be studied effectively due to harm to the individual, but the only existing observational study indicated a progression of up to 30 percent over 30 years.5 As with previous guidelines, the threshold for excision is CIN2+/HSIL, except in special circumstances such as pregnancy.
Special clinical circumstances. Pregnancy and immunosuppression require modification of the guidelines. Pregnant women will follow the risk-based guidelines with the provisions for colposcopy and treatment described above.
Immunocompromised. Immunocompromised individuals include those with HIV and solid organ transplant, allogeneic hematopoietic stem cell transplant, systemic lupus erythematosus, inflammatory bowel disease, or rheumatologic disease requiring immunosuppressive therapy. These individuals have unique cervical cancer screening guidelines, beginning within one year of the onset of sexual activity and continuing throughout a person’s lifetime. Screening intervals are annually for three years, then every three years up to age 30 with Pap tests only, switching to cotesting every three years after age 30. Referral to colposcopy is recommended for any cotest result of ASC-US/HPV+ or worse. If the Pap test is ASC-US without HPV testing, then repeating the Pap test in six to 12 months is recommended, with colposcopy referral for any abnormal result. Colposcopy is recommended for any positive HPV test, and any LSIL, ASC-H, atypical glandular cells, and HSIL cytology regardless of the HPV result.
Surveillance following HSIL treatment and hysterectomy. The guidelines recommend that individuals with a hysterectomy who have had HSIL (CIN2 or 3), AIS, or cancer continue to have HPV-based testing every three years for at least 25 years; this mirrors the guideline for surveillance in individuals treated by excision. Any person treated for HSIL should have more intense (six-month and then three annual) HPV-based surveillance tests.
Age over 65. Although women over 65 may generally exit cervical cancer screening programs, those who have a history of CIN2+, AIS, or cancer should continue surveillance screening as long as the person is in reasonable health, following the preceding guidelines. The recommended management for abnormalities follows the aforementioned guidelines. Data on screening in this age group is limited, but there is sufficient evidence of a higher rate of cervical cancer in individuals over age 656-7 to warrant extending screening beyond that age. If an individual without a history of cervical abnormality is screened beyond age 65, the preceding clinical management recommendations apply.
Primary HPV screening. Another major change from past guidelines is the recommendation to add an additional triage test, currently the Pap test, for all HPV-positive tests from primary HPV screening only, including those that are HPV16+ or HPV18+. If the primary HPV screening test is HPV16/18+ and a triage test is not feasible, the individual is referred to colposcopy, and a triage test is recommended at that time. Colposcopy and biopsies or expedited treatment are recommended for all HPV16+ or HPV18+ patients due to the high risk of CIN3+ in this cohort.
The intention is to improve specificity to allow for more effective patient triage. Pap tests will serve as a reflex test following a positive HPV result to determine which patients to refer to colposcopy. Adding a Pap test potentially provides information on the degree of precancer and can direct decisions on expedited treatment without biopsies. For example, the immediate risk of CIN3 in those who are HPV16+ and have HSIL on Pap tests exceeds the treatment threshold of 60 percent. In the future, it is possible that additional ancillary tests that enhance Pap test interpretation or rely on molecular methods will become acceptable for triage.
 Rare Pap test results and Pap test adequacy. Rare findings on Pap tests are difficult to fit into guideline recommendations, and data on their significance and appropriate follow-up is often minimal. One exception is the area of atypical glandular cells (AGC)/adenocarcinoma in situ (AIS), which has been widely studied over several decades. The problem with most studies of AGC is that morphologic criteria are poorly reproducible; cells may be of squamous, endocervical, or endometrial origin; and confirmatory biopsies are sometimes unavailable, making comparisons difficult. Conversely, AIS and AGC-favor neoplasia are associated with very high risk of precancer or invasion, regardless of HPV results,8 prompting the following recommendations.
Rare Pap test results and Pap test adequacy. Rare findings on Pap tests are difficult to fit into guideline recommendations, and data on their significance and appropriate follow-up is often minimal. One exception is the area of atypical glandular cells (AGC)/adenocarcinoma in situ (AIS), which has been widely studied over several decades. The problem with most studies of AGC is that morphologic criteria are poorly reproducible; cells may be of squamous, endocervical, or endometrial origin; and confirmatory biopsies are sometimes unavailable, making comparisons difficult. Conversely, AIS and AGC-favor neoplasia are associated with very high risk of precancer or invasion, regardless of HPV results,8 prompting the following recommendations.
Atypical glandular cells/adenocarcinoma in situ. Colposcopy with endocervical sampling is recommended for all Pap test results of AGC and AIS (despite qualifying terms such as “not otherwise specified” or “favor neoplasia”), regardless of HPV status, except for patients who are pregnant. Triage using HPV testing is not recommended, and triage with repeat Pap tests is unacceptable. If the patient is 35 years or older, or has increased risk for endometrial neoplasia (such as vaginal bleeding), then endometrial sampling is also indicated. If the Pap test is qualified as “atypical endometrial cells,” then initial biopsies of the endometrium and endocervix are preferred, but initial colposcopy is also acceptable. Colposcopy is recommended when endometrial and endocervical sampling is negative.
Subsequent surveillance recommendations for AGC without detected disease includes cotesting at 12 and 24 months, and if negative, cotesting at three years. Colposcopy is recommended for any abnormal results. For patients with Pap test reports of atypical glandular cells, favor neoplasia (AGC-N) or AIS without invasive disease detected on colposcopy and biopsy, a diagnostic excisional procedure is recommended, along with endocervical sampling.
Lack of transformation zone on Pap test. Patients 21 to 29 years of age who have a negative for intraepithelial lesion or malignancy (NILM) result may proceed with routine screening; it is unacceptable to use HPV testing as a triage test in these patients if the original intention was primary screening. For those ages 30 and older who have a NILM Pap test, insufficient transformation zone, and no or unknown HPV testing, HPV testing is preferred, but the Pap test may also be repeated in three years without HPV testing.
Review of recent studies found no adverse effect of the absence of a transformation zone on the detection of precancer.
Benign endometrial cells or benign glandular cells in post-hysterectomy individuals. Guidelines recommend that postmenopausal women with endometrial cells on Pap test have an endometrial biopsy. Post-hysterectomy individuals with benign glandular cells require no further evaluation, and no additional testing or surveillance is necessary for premenopausal individuals with benign endometrial cells, stromal cells, or histiocytes on Pap tests.
Unsatisfactory Pap test. Individuals with an unsatisfactory Pap test (with or without HPV testing) should have a repeat age-based screening test in two to four months, similar to prior guidelines, and using HPV testing to determine triage after an unsatisfactory Pap test is not recommended. For patients 25 years and older with an unsatisfactory Pap and positive HPV cotest, repeating the Pap test in two to four months or referral to colposcopy is acceptable. Colposcopy is recommended if genotyping reveals HPV16 or 18.
Management of histology results. The guidelines recommend that treatment decisions be made with patients and with regard for future cancer risk, childbearing risks, and personal preferences. The preferred management for histologic HSIL (CIN2 or CIN3) remains treatment, unless the patient is pregnant, but observation for CIN2 is acceptable if the patient has concerns about future pregnancy. Excisional therapy (LEEP, cold-knife cone, or laser cone) is preferred but ablation (cryotherapy, laser ablation, or thermoablation) is acceptable as long as the squamocolumnar junction is fully visualized, the lesion does not extend into the canal, and the endocervical sampling is not positive for CIN2+.
For CIN1 histology preceded by an HSIL Pap test, either a diagnostic excision or observation with colposcopy and HPV-based testing at one year is recommended. If CIN1 is preceded by an ASC-H result, observation at one and two years with HPV-based testing is recommended, and if an HSIL Pap test is reported or ASC-H persists at two years, diagnostic excision is recommended.
For individuals age 25 and older with persistent CIN1 (beyond two years), continual observation is preferred but treatment is acceptable. Those under age 25 should continue with observation.
Hysterectomy remains the preferred treatment for biopsy-proven AIS, but initial excisional procedure is recommended to exclude invasion, even if a hysterectomy is planned. Fertility-sparing procedures are acceptable if margins are negative and the patient can adhere to close surveillance.
How will these changes affect the laboratory? Currently, the two HPV testing platforms that the FDA approved for primary HPV tests in cervical cancer screening in individuals age 25 and older are the Cobas HPV Test (Roche) and BD Onclarity HPV Assay (Becton Dickinson). All other HPV testing platforms can be used for cotesting, reflex, surveillance, or follow-up testing, but their use is discouraged in primary HPV screening until FDA approved or further information about their safety is established. HPV RNA testing (Hologic Aptima) should be used in conjunction with Pap tests (cotesting) until more data become available. None of the current HPV platforms has FDA approval for testing vaginal specimens for any purpose, and laboratories doing so must validate their HPV platform for vaginal samples. Because most clinicians are unaware of federal regulatory requirements for instrumentation and testing platforms, laboratories should provide clear guidance and terminology on requisitions that permit users to distinguish primary HPV test orders from surveillance and reflex tests. Laboratory requisitions and online order entry options should be evaluated to determine if they are compliant with new management and screening guidelines.
The consensus process participants also considered the laboratory impact of proposed changes and addressed the recent CAP-ASCCP Lower Anogenital Squamous Terminology (LAST) project recommendations9 for lower anogenital tract pathology reporting and the use of p16. In recognition of the overuse of p16 and potential overdiagnosis of CIN2, 3 on surgical specimens,10 recommendations emphasize the judicious use of p16 to support a morphologic impression of CIN2,3, but they discourage upgrading a morphologic CIN1 based on a positive p16.
Although the LAST project advocates the use of “squamous intraepithelial lesion” terminology for surgical pathology as opposed to “cervical intraepithelial neoplasia,” it currently recommends qualifying all HSIL surgical results with the qualifier -CIN2 or -CIN3 to improve future epidemiologic studies and tailor clinical management. The low rate of progression of CIN2 allows for longer surveillance intervals rather than requiring excision, thereby reducing the risk of cervical incompetence in younger women with this lesion.11,12 Current recommendations allow for more conservative management of individuals under age 25 using observation only.
-
- Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24(2):102–131.
- Egeman D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Genit Tract Dis. 2020;24(2):132–143.
- Wentzensen N, Massad LS, Mayeaux EJ Jr, et al. Evidence-based consensus recommendations for colposcopy practice for cervical cancer prevention in the United States. J Low Genit Tract Dis. 2017;21(4):216–222.
- Committee on Practice Bulletins–Gynecology. Practice bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–434.
- Gravitt PE, Landy R, Schiffman M. How confident can we be in the current guidelines for exiting cervical screening? Prev Med. 2018;114:188–192.
- Feldman S, Cook E, Davis M, et al. Cervical cancer incidence among elderly women in Massachusetts compared with younger women. J Low Genit Tract Dis. 2018;22(4):314–317.
- Zhao C, Florea A, Onisko A, Austin RM. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: results from a large academic womens hospital laboratory employing sensitive screening methods. Gynecol Oncol. 2009;114(3):383–389.
- Darragh TM, Colgan TJ, Cox JT, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012;16(3):205–242.
- Castle PE, Adcock R, Cuzick J, et al. Relationships of p16 immunohistochemistry and other biomarkers with diagnoses of cervical abnormalities: implications for LAST terminology. Arch Pathol Lab Med. 2020;144(6):725–734.
- Silver MI, Gage JC, Schiffman M, et al. Clinical outcomes after conservative management of cervical intraepithelial neoplasia grade 2 (CIN2) in women ages 21-39 years. Cancer Prev Res. 2018;11(3):165–170.
- Moscicki A-B, Ma Y, Wibbelsman C, et al. Rate of and risks for regression of a cervical intraepithelial neoplasia 2 in adolescents and young women. Obstet Gynecol. 2010;116(6):1373–1380.
Dr. Crothers is associate professor of pathology practicing subspecialty gynecologic, breast, and cytopathology consultation at the Joint Pathology Center, Silver Spring, Md. She is a former chair and former member of the CAP Cytopathology Committee. Dr. Davey is professor of pathology and associate dean at the University of Central Florida, Orlando. She practices pathology at the Orlando VAMC and is a former CAP Cytopathology Committee member.