Christine N. Booth, MD
Rhona J. Souers, MS
Jennifer A. Brainard, MD
May 2019—The CAP regularly surveys the practices of the laboratories participating in the CAP Nongynecologic Cytopathology Education Program, or NGC. Members and staff of the CAP Cytopathology Committee developed a supplemental questionnaire eliciting feedback on pathologist competency activities. The Survey was mailed to 2,142 participants in the NGC-B 2018 education program. The pathologist competencies queried were as follows:
- Performance of a secondary review prior to sign-out of a first-time diagnosis of malignancy.
- Investigation of corrected/amended reports in regard to monitoring the number of reports, as well as
- Classifying the reasons for the amendment.
- Investigation of discrepancies between rapid on-site adequacy assessment and final diagnosis.
- Tracking of specific nongynecologic diagnostic category usage rates over time.
In addition to activity performance, information was collected on the level at which the activity was monitored and documented and whether individual performance was compared with a benchmark or laboratory average. Of the 2,142 participating laboratories, 837 laboratories responded, for a 39 percent response rate. Fig. 1 shows the percentage of laboratories performing the five pathologist competency activities.
Seventy-seven percent (644 of 831) of laboratories perform a secondary review before the sign-out of a first-time diagnosis of malignancy. Of the 644 laboratories, 604 provided the level at which the activity is monitored and documented. Seventy-nine percent monitor at the individual level, 37 percent monitor at the laboratory level, and 16 percent monitor and document both the individual and laboratory performance of this activity. In addition, slightly more than half of the laboratories (53 percent) that monitor and document individual performance reported that they compare the individual’s performance with either a benchmark or a laboratory average.
Eighty-five percent of laboratories monitor the number of corrected/amended reports. Of the 696 laboratories that responded to the question about the level at which this activity is monitored and documented, 68 percent say they monitor by individual, while 55 percent monitor and document at the level of the cytopathology laboratory as a whole. Almost one quarter of the laboratories (159) monitor both the individual and laboratory number of corrected/amended reports.
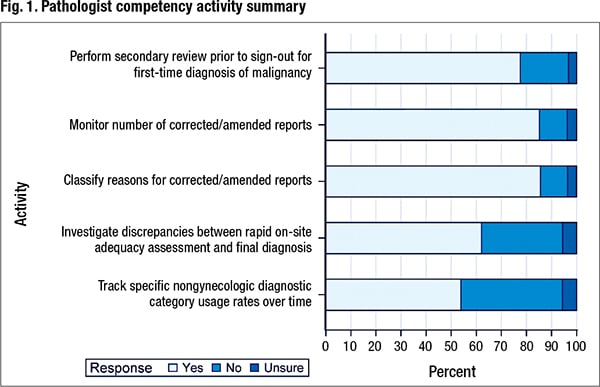 Of the 826 laboratories that responded to the question about whether they classify the reasons for an amended report, 86 percent (707) say they classify the reasons. Of 673 responses about the level at which this activity is monitored and documented, 68 percent of laboratories (461) say they monitor this at the level of the individual; 53 percent (356) monitor and document at the level of the laboratory. Twenty-one percent (144) monitor both the individual and laboratory in classifying the reasons for a corrected/amended report. Of 391 laboratories that responded to the question about whether individual performance was compared with a benchmark or a laboratory average, 65 percent (253) indicated they did so.
Of the 826 laboratories that responded to the question about whether they classify the reasons for an amended report, 86 percent (707) say they classify the reasons. Of 673 responses about the level at which this activity is monitored and documented, 68 percent of laboratories (461) say they monitor this at the level of the individual; 53 percent (356) monitor and document at the level of the laboratory. Twenty-one percent (144) monitor both the individual and laboratory in classifying the reasons for a corrected/amended report. Of 391 laboratories that responded to the question about whether individual performance was compared with a benchmark or a laboratory average, 65 percent (253) indicated they did so.
Eight hundred ten labs responded to the question about whether they investigate discrepancies between rapid on-site adequacy assessment and the final diagnosis. Sixty-two percent (503) say they are performing this activity; 32 percent (262) are not. Slightly over 5.5 percent (45) of respondents were unsure whether their laboratories routinely perform this investigation. Of 488 respondents that shared the level at which the activity is monitored and documented, 78 percent (383) indicated this activity is monitored and documented at the level of the individual; 40 percent (196) indicated that the investigation of discrepancies between rapid on-site evaluation and final diagnosis was monitored and documented at the level of the laboratory. Nineteen percent (91) monitor and document the discrepancies between rapid on-site adequacy assessment and final diagnosis at the individual and at the laboratory level. Sixty-one (204) percent of 335 laboratories also responded that they compare individual performance with a benchmark or the laboratory average in the investigation of these discrepancies.
The final competency activity that was queried in this survey was the tracking of specific nongynecologic diagnostic category usage rates over time. Fifty-four percent of the laboratories (446 of 828) say they do track the use of specific nongynecologic diagnostic category rates over time; 40 percent (335) do not. Six percent (47) of respondents were unsure. Of the 439 respondents that indicated the level at which this tracking of nongynecologic diagnostic category usage rates over time is performed, 53 percent (233) of laboratories said they monitor and document this at the level of the individual; 78 percent (341) monitor and document at the level of the laboratory. Thirty-one percent (135) monitor it at the individual and at the laboratory level. Of 211 laboratories that responded to an additional question about whether an individual’s performance is compared with a benchmark or laboratory average, 83 percent (176) monitor individual pathologists to either a benchmark or a laboratory average.
The most frequently performed activities were a secondary review before sign-out for a first-time diagnosis of malignancy and both corrected/amended report activities, with 63 percent (526 of the 837 laboratories) performing all three activities. Additional analyses were performed to determine whether there were differences in the participation in pathologist competency activities by institution type. The only activity that showed a significant difference by this practice characteristic was the investigation of discrepancies between rapid on-site adequacy assessment and final diagnosis (Chi-square test; P < .001). Regional/local independent laboratories and national/corporate laboratories perform this activity less frequently than other institution types. Of the 38 national/corporate laboratories, only 37 percent (14) investigate these discrepancies, and only about half (35) of the 71 regional/local independent laboratories perform the investigations. These rates are significantly lower than for other practice types where 70 percent of laboratories (367 of 524) investigate the discrepancies between rapid on-site adequacy assessment and final diagnosis.
In summary, there is little available data regarding pathologist competency assessment, which prompted this CAP survey. Survey results highlight a variety of pathologist competency activities currently performed in addition to variable practices for monitoring and documenting these activities. The results provide a framework for a future study.
Dr. Booth, chair of the CAP Cytopathology Committee, is co-section head of cytopathology, Cleveland Clinic. Souers is a CAP senior biostatistician. Dr. Brainard, vice chair of the Cytopathology Committee, is vice chair of cytology operations, Cleveland Clinic.