
Andrew S. Field, FRCPA, FIAC, DipCytopath(RCPA)
January 2018—In countries with developed medical infrastructure, the use of breast fine-needle aspiration biopsy (FNAB) cytology has had its share of challenges over the past 20 years, among them the use of core needle biopsies. In developing countries where the use of FNAB cytology has been increasing rapidly, breast lesions are one of the most common sites sampled by FNAB. In 2016, the International Academy of Cytology Executive Council put together a “Breast Group,” which consists of cytopathologists, surgical pathologists, radiologists, surgeons, and oncologists working in breast care, with the aim of producing a comprehensive and standardized approach to breast FNAB cytology reporting.1,2
This approach will address the current challenges to FNAB cytology and include best-practice guidelines for the indications for breast FNAB cytology and the techniques of FNAB, smear making, and material handling. It will include a practical, standardized reporting system, including report content requirements, with defined descriptive terms and categories, structured reports with checklists and formats, and recommendations for the use of ancillary diagnostic and prognostic tests and suggested management algorithms. A standardized approach with best-practice guidelines will improve FNAB and smear-making technique, training, routine reporting, and quality assurance programs. If linked to management recommendations, it will improve clinicians’ understanding and use of FNAB cytology services.
Why a new reporting system?
Since the 1996 National Cancer Institute consensus meeting made recommendations for breast FNAB cytology reporting, there have been many developments in the diagnostic workup of breast lesions in surgeons’ rooms, breast clinics, and mammographic screening program assessment clinics, including the use of tomographic mammography, ultrasound, and MRI.3 There have also been significant developments in the role of various diagnostic procedures in management algorithms, and the use of breast FNAB cytology now varies greatly between breast clinics for symptomatic women, mammographic screening program assessment clinics, and hospitals in various cities and states as well as between developed and developing countries.
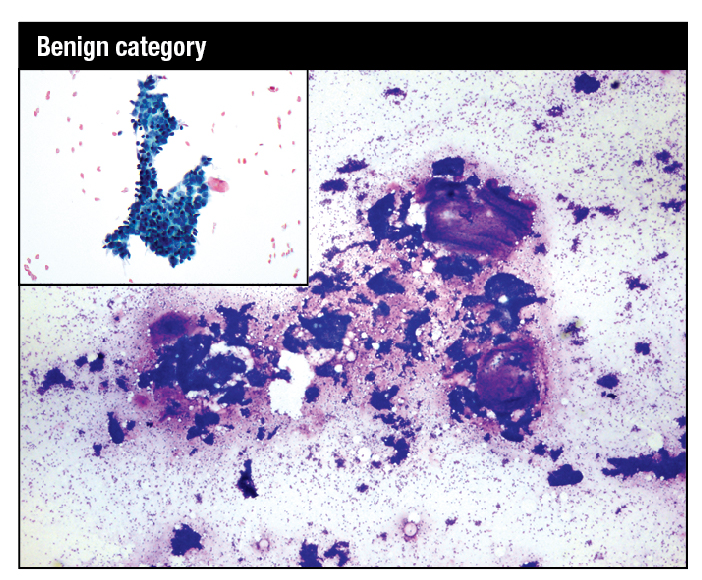
Modified Giemsa stain. Highly cellular smear showing fibroadenoma with mix of small and large hyperplastic ductal epithelial cell tissue fragments and large myxoid stromal fragments. High-power image shows myoepithelial nuclei on the epithelial fragments and in the background as bare bipolar nuclei. Inset: Modified Giemsa. Fragment of benign breast tissue consisting of ductal epithelial cells with interspersed myoepithelial cells.
Breast FNAB cytology does offer many advantages because it is quick, is minimally invasive, causes minimal physical and psychological discomfort, and is acceptable to patients. It is a relatively inexpensive test. It enables rapid onsite evaluation (ROSE) and provisional reporting, which is ideal for multidisciplinary “one stop” diagnostic clinics that provide same-day clinical, radiological, and provisional cytological assessment.
Breast FNAB is a highly specific and sensitive test to accurately diagnose benign and malignant lesions when undertaken by an operator experienced in the biopsy technique and cytopathologists experienced in reporting breast cytology. It is cost-effective for the preoperative diagnosis of palpable and ultrasound-detected impalpable breast lesions. It can also provide formalin-fixed, paraffin-embedded cell blocks for immunohistochemistry for prognostic indicators, including estrogen and progesterone receptors and for in situ hybridization for HER2. Material from directly smeared slides or cell block material can be used for PCR and other potential molecular testing.4
In medically under-resourced developing countries, where more than 80 percent of the world’s population lives, breast FNAB is the test for all palpable lesions, in a setting where preoperative imaging and core needle biopsy and histopathology are not readily available and are expensive options.5,6
Challenges and solutions
The greatest challenge now to breast FNAB cytology is the quality of the FNAB procedure and of the smearing technique. These are crucial to a successful breast cytology service and the major source of quality assurance problems with breast FNAB. Poor performance of the FNAB and the direct smear are the elephant in the room in any discussion of the role of breast cytology.2
Currently, radiologists and their trainees perform the majority of breast FNAB in the developed world, rather than cytopathologists. There is a long and successful tradition of cytopathologists carrying out FNAB of breast palpable lesions. Cytopathologists are immediately aware of the quality of their technique because they are reporting the slides, while radiologists often have minimal contact with the reporting pathologist. FNAB is regarded as a simple test, but it requires good training and ongoing experience with constant monitoring of the diagnostic yield and adequacy rates. The number of FNAB of breast has decreased in the developed world, resulting in fewer training opportunities for trainee radiologists and pathologists and a lack of adequate training. This has led to a general decrease in the quality of breast cytology specimens.
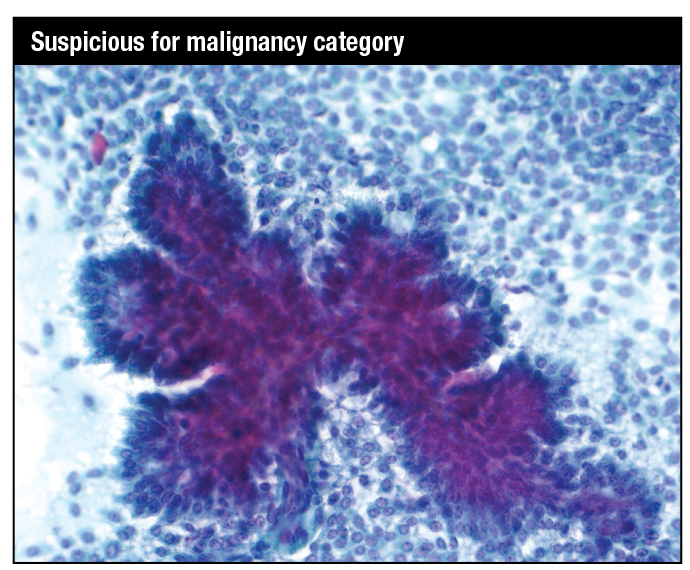
Papanicolaou stain. Papillary tissue fragment with fibrovascular core covered in multilayered atypical cuboidal to columnar epithelium with moderately pleomorphic nuclei in a background of dispersed single cells showing moderate nuclear atypia: specifically, suspicious for papillary intraduct carcinoma, with or without an invasive component.
The radiologist uses ultrasound guidance for palpable and impalpable lesions, which has increased the range of accessible lesions but at the same time accentuated the problems in the performance of the FNAB and in making smears. The key elements in a breast FNAB are the fixation of the specimen and a rapid technique. Ideally, the needle should be introduced for fewer than 10 seconds, with 10 to 15 rapid passages of the needle into and just through the lesion, using the cutting action of the needle bevel. For cytopathologists and radiologists, ultrasound can be helpful in assessing palpable lesions. But it is more difficult to fix a palpable breast lesion when an ultrasound probe is present, and the time the needle is in the lesion, the “dwell time,” is lengthened, leading to an increased incidence of blood contamination and clotting of the material in the needle. Further, if aspiration is applied early in the FNAB without the cutting action of the needle having been utilized, the result may be inadequate, hemodiluted, and obscured material.
The second crucial preanalytical step is preparing direct smears, and poorly trained operators or their assistants can ruin good material by using poor smear-making technique. Liquid-based preparations do avoid poor smearing technique and the air drying of alcohol-fixed material, but they prevent ROSE, decrease the crucial pattern recognition diagnostic features in breast cytology, and increase expense.

Modified Giemsa stain. Carcinoma of the breast. Small tissue fragments of crowded cells with highly atypical nuclei and eccentric cytoplasmic vacuoles in some cells, and similar dispersed single cells.
ROSE carried out by a cytopathologist or well-trained cytotechnologist attending the FNAB procedure lowers inadequacy rates, makes it possible to provide an immediate provisional report to the clinician and patient, improves the quality of direct smears and the triaging of material for expensive ancillary tests, and decreases the costs of patient recalls and second procedures. Most important, ROSE provides immediate contact between the cytopathology team and radiologist. There can be constant interaction between the cytopathologist viewing the slides and the proceduralist, which leads to better quality FNAB material and reporting and better breast care for the patient. Ideally, the cytopathologist can perform the FNAB using ultrasound, if necessary, for palpable lesions or to target an impalpable lesion found by imaging. If this is not possible, a radiologist or other clinician willing to develop his or her technique and work with the cytopathologist to achieve better results should perform the FNAB.7
There are analytical challenges in interpreting breast cytology slides, and for inexperienced pathologists this is particularly so. Breast FNAB cytology requires specific training and continuing exposure to a significant caseload, just as in any other specialty area of cytology or surgical pathology. The reduction in the number of cases in most teaching hospital programs has led to a reduction in the level of training in breast cytopathology. In breast cytology, high cellularity and dispersal do not necessarily mean malignancy, and proliferative lesions and intraductal and even invasive carcinomas can have overlapping features. Distinguishing intraductal and invasive carcinomas can be difficult.

Modified Giemsa stain. Moderately cellular smear showing a large mildly hypercellular stromal fragment containing a branching capillary and with an adjacent tissue fragment of ductal epithelial cells in a background of stripped nuclei, raising the possibility of a low-grade phyllodes tumor.
Core needle biopsy (CNB) in some parts of the developed world has virtually replaced breast FNAB, particularly in mammographic screening program assessment clinics, where a significant proportion of the cases involve workup of calcifications, and in the follow-up of any abnormal mammogram in general breast work. Breast core reporting is part of most surgical pathology practices and the core biopsy technique is relatively standard, so no special training is required. Further, the screening program experience in the use of FNAB and CNB has been inappropriately extrapolated into the assessment of all breast lesions, whether palpable or not, in clinical breast units. There is a need to establish and recommend the most appropriate use of these two complementary tests. CNB is more invasive with a greater rate of complications and is not required in many cases in the general breast clinic where the vast majority of lesions will be cysts, fibrocystic change, fibroadenomas, and other mass lesions including a small number of carcinomas. Breast FNAB, particularly when ROSE is available, can be used to triage the cases that do require CNB, leading to reduced costs. CNB is more expensive in terms of the biopsy equipment and the histopathology processing and reporting, and as such is inappropriate in a low-resource setting.5,6
Standardized reporting system
It is in this environment of the changing role of breast FNAB cytology that the members of the IAC Breast Group are developing a standardized reporting system. Small groups have prepared draft reviews and statements on the technical aspects of the FNAB; the different diagnostic categories used in reporting cases, including definitions, suggested terminology, and risk of malignancy based on positive and negative predictive values; and on the appropriate current ancillary testing role. These drafts have just been distributed to all members of the group for discussion. A consensus will be reached and draft documents will then be published on the IAC website early this year. Cytopathologists and clinicians will have the opportunity to critique and discuss the drafts, and the group will address their comments and modify the drafts where appropriate. Discussions will then be held with other cytology organizations to achieve, if possible, an international consensus. The rationale for the reporting system was presented at a number of cytology meetings in 2016 and 2017. The final documents will be published this year, and an atlas will be published by end of the year.
The standardized approach will include best-practice guidelines for the FNAB and smear-making techniques and the structure of reports. Structured reporting improves the quality, clarity, and reproducibility of reports across departments and between states and countries, and it will improve patient management and facilitate research and quality assurance measures.8 Standardized use of cell blocks, IHC, ISH, and other molecular tests of prognostic and diagnostic markers will improve care and reduce costs.
Structured reports establish a format with standard headings, definitions, and common terminology and include required information, which can be either a mandatory standard or a recommended guideline.9 They are usually based on a checklist that matches the workflow of the laboratory and cytopathologist and are presented in a clear format that conveys information across borders to pathologists and to clinicians.
The FNAB cytology report should resemble a breast core or any other surgical report and include: minimum data requirements, which can include a statement of whether the lesion is completely benign; a statement of cellularity, which is a measure of the adequacy of the material; a cytological description, which should include key cytological criteria; and a conclusion or summary using a standardized descriptive terminology diagnosis. This conclusion should be as specific as possible or, if a specific diagnosis is not possible, provide a weighted differential diagnosis based on the cytological criteria present. A code or category can be part of the body of the report and is useful for quality assurance and research, but a simple number should never be used in isolation or as a conclusion, as it will impair the clinician’s understanding of the individual report. The key to the report is a clear, descriptive diagnosis using standardized terminology.
Five-category system
The Breast Group has decided to use a five-category system used widely internationally: category 1: insufficient material; category 2: benign; category 3: atypical, probably benign; category 4: suspicious for malignancy, probably in situ or invasive carcinoma; and category 5: malignant.
There has been discussion around the use of the terms “insufficient” or “inadequate” for cases that lack epithelium, such as cyst contents, and around the definitions of “atypical” and “suspicious for malignancy” and the various situations when these terms should be used. The decision was made to retain an “atypical” category, which allows for a high NPV for a benign diagnosis, and a “suspicious for malignancy” category, to maintain a high PPV for a malignant diagnosis—and these two categories allow for stratification of the risk of malignancy. The causes of an “atypical” cytological diagnosis include technical problems with the FNAB and the smear making, scant material and interpretive problems related to the inherent characteristics of the lesion, or a combination of these factors intertwined with the experience of the cytopathologist. The causes of a “suspicious for malignancy” diagnosis are similar and should always be stated in the report along with the specific lesion the smears are suspicious of.
A structured reporting system requires checklists of key cytological features for specific lesions that are based on an analytical approach using low-power pattern recognition combined with high-power cytological features integrated in a final diagnosis.10
The FNAB cytology report is used in conjunction with the clinical and imaging findings in the triple-test approach, which yields very high PPV and NPV and provides the basis for management decisions. The Breast Group will establish best-practice protocols for the suggested management of each of the five categories with their varying risks of malignancy, while taking into account the vast differences between the developed and developing world in the potential availability of imaging, CNB, surgical pathology, and management options. These best-practice guidelines will include the indications for and role of FNAB and CNB in the management algorithms and allow for the great variations in medical infrastructure.5,6
For example, the current draft document suggests an “atypical” report should lead to an immediate reassessment of the imaging and clinical findings. If the triple test is negative apart from the atypical cytology report, a decision can be made to simply review the patient at a shortened time interval. Or if the imaging or clinical findings are indeterminate, immediate CNB can be performed. Where CNB is not available, repeat FNAB or possibly excision biopsy can be the management option. On the other hand, a “suspicious” cytology report requires a mandatory biopsy, which can be a repeat FNAB but is usually a CNB if available, or in some situations a simple excision biopsy.
Members of the International Academy of Cytology Standardized Reporting of Breast FNAB Cytology Group hope that cytopathologists and cytotechnologists will review the draft proposals and provide their input once the proposals are placed on the IAC website.
- Field AS, Schmitt F, Vielh P. IAC standardized reporting of breast fine-needle aspiration biopsy cytology. Acta Cytol. 2017;61(1):3–6.
- Field AS. Breast FNA biopsy cytology: current problems and the International Academy of Cytology Yokohama standardized reporting system. Cancer Cytopath. 2017;125(4):229–230.
- Abati A, Abele J, et al. The uniform approach to breast fine-needle aspiration biopsy. Diagn Cytopathol. 1997;16(4):295–311.
- Schmitt F, Vielh P. Fine-needle aspiration cytology samples: a good source of material for evaluating biomarkers in breast cancer. Histopathology. 2015;66(2):314–315.
- Masood S, Vass L, Ibarra JA Jr, et al. Breast pathology guideline implementation in low- and middle-income countries. Cancer. 2008;113(8 suppl):2297–2304.
- Anderson BO. Fine-needle aspiration for breast cancer diagnosis: one size does not fit all. J Natl Compr Canc Netw. 2016;14(5):599–600.
- Ljung BM, Drejet A, Chiampi N, et al. Diagnostic accuracy of fine-needle aspiration biopsy is determined by physician training in sampling technique. Cancer. 2001;93(4):263–268.
- Ellis DW, Srigley J. Does standardised structured reporting contribute to quality in diagnostic pathology? The importance of evidence-based datasets. Virchows Arch. 2016;468(1):51–59.
- Structured Pathology Reporting of Cancer. Royal College of Pathologists of Australasia website. https://www.rcpa.edu.au/Health-Care-Professionals/Structured-Pathology-Reporting-of-Cancer.
- Field AS, Zarka MA. Chapter 5: Fine needle aspiration biopsy cytology of breast: a diagnostic approach based on pattern recognition. In: Practical Cytopathology: A Diagnostic Approach to Fine Needle Aspiration Biopsy. Philadelphia: Elsevier; 2017.
[hr]
Dr. Field is an associate professor, Notre Dame University Medical School, Sydney, Australia, and a cytopathologist and surgical pathologist, Department of Anatomical Pathology, St. Vincent’s Hospital, also in Sydney. Suspicious and malignant category images supplied by Dr. Field. Benign and atypical category images supplied by Kristen Natale, DO, medical director of cytopathology, Walter Reed National Military Medical Center, Bethesda, Md., and Timothy Harkom, DO, a fourth-year pathology resident at WRNMMC. Dr. Natale and Dr. Harkom are members of the CAP Cytopathology Committee.