Karen Titus
December 2021—There are success stories. There are overnight success stories. And then there are things that just seem to happen overnight—minus the success.
In the midst of chronic discontent over the use of a race coefficient in equations for estimating glomerular filtration rate, one San Francisco hospital sought to make a change. The hope was to help end disparities in health care, such as lower kidney transplantation rates in Black people with chronic kidney disease.
The change at Zuckerberg San Francisco General Hospital was pushed by what Neil Powe, MD, MPH, MBA, calls “a very small group of faculty and trainees lobbying the lab, unbeknownst to others.” The lab reported two values, using the eGFR equation with and without the race coefficient, but assigned “high muscle mass” and “low muscle mass” to the two values as a way to step around the troubling race component.
Dr. Powe, the hospital’s chief of medicine, doesn’t mince words when he considers how the change was made. “And it stereotyped ethnic groups even more,” he says.
Despite all the talk and desire to do things better—which reached a nationwide crescendo in the summer of 2020—there remained misapprehension about the role race has played in determining eGFR. Change was needed, says Dr. Powe, who is also the Constance B. Wofsy distinguished professor and vice chair of medicine, University of California, San Francisco. “But we needed a pathway to replacement.”
That path has opened up, recently and widely, with a new eGFR equation recommended by the National Kidney Foundation-American Society of Nephrology Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease (Delgado C, et al. Am J Kidney Dis. Published online ahead of print Sept. 23, 2021. https://doi.org/10.1053/j.ajkd.2021.08.003). Dr. Powe co-chaired the task force, along with UCSF colleague Cynthia Delgado, MD, associate professor and associate chief of nephrology–clinical operations, Department of Medicine, UCSF, and nephrology section, San Francisco VA Medical Center.
The equation is one of three recommendations the task force made:
- Immediate implementation of the CKD-EPI creatinine equation refit without the race variable. As the authors note, the equation “does not include race in the calculation and reporting, includes diversity in its development, is immediately available to all labs in the U.S., and has acceptable performance characteristics and potential consequences that do not disproportionately affect any one group of individuals.”
- National efforts to increase routine, timely use of cystatin C, especially to confirm eGFR in adults with or at risk for CKD. Combining creatinine and cystatin C is more accurate, the task force notes, and supports better clinical decisions than using one filtration marker alone.
- Further research, aimed at eGFR estimation with new endogenous filtration markers and interventions to eliminate race and ethnic disparities in care.
The task force’s work can be seen as sort of a D-Day-esque effort to tackle longstanding problems in eGFR measurements. Every boat is being launched, from skiff to schooner.
The task force held 40-plus sessions to assemble and review data and evidence; in 16 sessions it heard testimony from 97 experts. It also took testimony from the community at large, including students and trainees; clinicians, scientists, and other allied health professionals; and patients, family members, and others—450 people in all.

Dr. Neil Powe and Dr. Cynthia Delgado, co-chairs of the task force that recommends use of the CKD-EPI creatinine equation refit without the race variable. “I want this work to stand,” Dr. Delgado says of the task force recommendations. “And I want this work to reflect that this is not a perfect test.” (Photo: Cindy Charles)
The recommendations are based on the work of another group (Dr. Powe, among others, was part of both groups), the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), which formulated and cross-validated new GFR estimating equations without a race coefficient (age and sex are the only demographic factors), comparing them to currently used CKD-EPI equations (Inker LA, et al. N Engl J Med. 2021;385[19]:1737–1749). The group used two data sets to develop current equations. One was from the CKD-EPI 2009 for eGFRcr (10 studies, 8,254 participants, 31.5 percent Black); the other from CKD-EPI 2012 for eGFRcys and eGFRcr-cys (13 studies, 5,352 participants, 39.7 percent Black) for both serum creatinine and cystatin C. They then compared the accuracy of the new eGFR equations to measured GFR in a validation set of 12 studies, seven of them new (4,050 participants, 14.3 percent Black).
Explains lead author and task force member Lesley Inker, MD, MS: “The work we did allowed the task force to come to this conclusion. Recommendation No. 1, of the CKD-EPI equation refit without the use of race, is the equation as reported by the New England Journal on the same day.” Dr. Inker is director of the Kidney and Blood Pressure Center and of the Kidney Function and Evaluation Center, Tufts Medical Center, and associate professor of medicine, Tufts University School of Medicine.
Among the criteria upon which the NKF-ASN task force’s recommendations were based were key elements of interest to labs, she says, including widespread availability of the filtration marker in all clinical laboratories, standardization of the assays, and high-throughput analyzer capability. The new approach would need to be relatively easy for laboratories to adopt.
Any new approach the task force developed, moreover, would need to be based on diverse populations. Of 12 approaches that did not include race as a variable, for example, seven were developed using populations that included no Black individuals. Among six approaches that were developed in a diverse population, the task force chose five for more in-depth analysis.
The task force also took into account performance in external validation (in terms of bias, precision, and accuracy), as well as potential long-term consequences.
“I think they did a very good job of rolling this out in a comprehensive way,” says Melanie Hoenig, MD, associate professor of medicine, Harvard Medical School, Renal Division, Beth Israel Deaconess Medical Center. The key reports “all came out online at once,” she says. “And the National Kidney Foundation’s website, kidney.org, updated their calculators at the same moment.” She also lauds the comprehensive supplementary data included as part of the task force report.
All this is aimed at improving a measurement that can seem dicey from a clinical standpoint.
One may be the loneliest number, but a collateral point might also be true: One number alone may not be sufficient. A single eGFR value, even calculated with the best of all possible equations, is not the beau ideal. Many physicians, Dr. Hoenig says, “probably don’t even remember that it’s an estimate. They’re just looking for a number to help guide them in terms of medication.”
Nevertheless, eGFR has pulled the lion’s share of attention, even more so with the latest reckonings on the race coefficient. Given that spotlight, observers are hoping the new equation and recommendations will change not only what labs and clinicians do but also how they talk about what they’re doing, with one another and with their patients. Those working to bring about change dug deep; the hope is that the change will be equally seismic.
Dr. Delgado runs the low kidney function clinic as well as the dialysis unit at the San Francisco VA. The hospital intends to implement the new equation; it already offers cystatin C in-house.
In one regard, the new equation may not change her day-to-day practice significantly, she says. “I have a tendency to follow change in kidney function,” she says. “And there are so many other things that one evaluates in a person when you’re trying to decide whether they’re going to need dialysis,” including metabolic abnormalities, proteinuria, and albumin in urine.
But on an equally practical level, she continues, “this change addresses any questions and ambiguity” that have bubbled up with the current estimating equation. The race coefficient “didn’t make it easy to have a dialogue with patients.”
In that regard, the new eGFR equation might serve almost as a type of medical talking stick. “It’s a moment for patients and practicing physicians to have a better relationship because it clarifies the dialogue a little bit better,” Dr. Delgado says. “Patients are no longer going to be apprehensive or worried that their kidney function estimation and their [treatment] plan are going to be driven by how they look. As a clinician, that’s a great thing.”
The new eGFR equation looks familiar, and even similar to the CKD-EPI equation currently in use—though it lacks the race coefficient—“but it’s different,” says Dr. Hoenig. “There are lots of little parts of it that are different. The formula itself is really complicated, with several exponents.”
Many clinicians won’t dip far into the backstory of how the new equation was developed, Dr. Hoenig says. “They’re looking for the lab report, they’re looking for the creatinine, and then they’re looking for whatever the lab says is the estimated GFR.”
Nevertheless, she suggests it’s worth peering more closely at the work behind the new formula. Fig. 1 in the New England Journal study provides a strong visual of how the current and the new creatinine and creatinine-cystatin C equations perform in Black and non-Black populations. It also looks at the performance of the current cystatin C equation, alone, in both groups.
The current CKD-EPI works well for non-Blacks. “It doesn’t mean every single person is on the line of identity, but on a population level the formula works well,” Dr. Hoenig says. For non-Blacks, the P30 is 89 percent; that is, 89 percent of participants are within 30 percent of their true GFR. (In Dr. Hoenig’s experience, “People are shocked by that info: This eGFR is only within 30 percent up or down of the number you’re giving me? But providers don’t want that nuance. Primary providers have increasingly challenging work addressing complex medical conditions,” she says, “and are grateful for dosing guidelines linked to a single eGFR value.”)
Correct classification shows how many people fall into the correct stage of CKD predicted by the eGFR, Dr. Hoenig continues. For non-Black participants, that figure is 69 percent.
For Black people, using the current formula with the race coefficient results in more dismal numbers. The P30 is 85 percent, and the correct classification is 63 percent.
The authors also looked at the impact of simply removing the race coefficient from the current CKD-EPI formula and keeping the other elements the same. Simply put, it doesn’t work.

Dr. Hoenig
“This is the argument that many have been making—that you can’t just drop the race coefficient,” Dr. Hoenig says. “Because then you will misclassify more people.” For Black participants, the correct classification falls to 59 percent when the coefficient is dropped; for non-Blacks, the correct classification is unaffected and remains at 69 percent. “Honestly, none of these numbers are actually very good.”
The new formula yields results that fall in between those two approaches. For Black participants, the P30 is 87 percent. Correct classification is 62 percent. For non-Black participants, P30 drops to 86 percent; correct classification is 67 percent.
Bias (the median difference between measured GFR and eGFR) for Black participants is +3.6 milliliters per minute per 1.73 square meters with the new equation, which falls in between the numbers for the current equation (bias: –3.7) and the current equation dropping the race coefficient (bias: +7.1). (A positive sign indicates underestimation of measured GFR; a negative sign, overestimation.) For non-Black participants, the bias in the new equation is now –3.9; in the other two equations, it’s –0.5. “But, on the whole, for an individual it does not perform very differently from before,” says Dr. Hoenig. “And now we have a much better profile for the Black participants.” On average, the new equation is an improvement, she says.

Dr. Inker
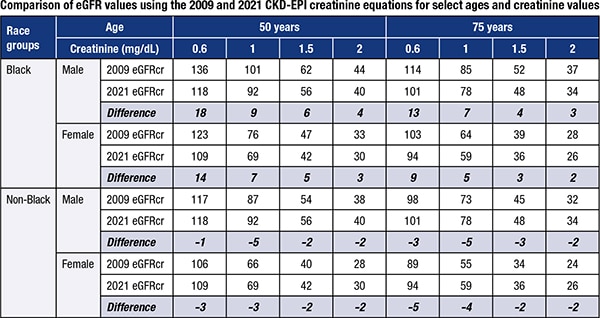
The new eGFRcr equation has similar overall performance to the current equation, but its main advantage, Dr. Inker says, is that it does not disproportionately affect any one group of individuals. The true GFR has not changed, even though the reported value might differ—which is important for patients and health care professionals to remember. “In general, the new eGFR values will be slightly lower in Blacks and slightly higher in non-Blacks than previously reported values, and the changes will be greater for younger patients and those with higher levels of GFR,” Dr. Inker says (see table).
Dr. Hoenig applauds the supplemental material that identifies the makeup of each cohort that was used in equation creation. “One of the things that’s always been murky—since race is a social construct—is how participants were assigned.”
When the MDRD GFR equation was developed, “that notion of how people were assigned to each group was not generally considered,” Dr. Hoenig says. When the current CKD-EPI was developed, things weren’t much better. “You cannot find that information when you read that report.” To do so requires pulling the 16 studies in the development portion and the 10 in the validation cohort, “and then trying to figure out how they recruited patients.”
Dr. Hoenig extols the transparency by everyone involved. Those who developed the new equation “pulled the curtains back on that, which I think is helpful.”
Fig. 1 in the New England Journal article provides similar insight into how adding cystatin C to the various equations performs. Similar patterns of improvement are evident as well. Adding cystatin C to creatinine improves the accuracy of the estimated GFR for both groups, with less difference between groups.
There was no need for a new equation that included only cystatin C, since the current one does not use a race coefficient. The biggest change may simply be the push to use it more in combination with creatinine so the result is more accurate, Dr. Inker says.
For the vast majority of laboratories, cystatin C is a send-out; it will require what Dr. Hoenig calls “activation energy” to bring it in-house.
 There is a “very real logistical hurdle to incorporating cystatin C,” agrees Jonathan Genzen, MD, PhD, chief operations officer, ARUP Laboratories, and associate professor (clinical), University of Utah Department of Pathology. It can be performed on many automated analyzers, “so I would not describe it as a particularly esoteric test. Rather, it is not yet available on the extensive array of automated platforms that you would want at this point.” Ideally, it would be performed on combined chemistry-immunology instrument clusters so that cystatin C and creatinine can be performed at the same time without specimens having to be distributed among laboratory sections, he says. “But cystatin C is not yet in widespread use, and it adds significant cost.”
There is a “very real logistical hurdle to incorporating cystatin C,” agrees Jonathan Genzen, MD, PhD, chief operations officer, ARUP Laboratories, and associate professor (clinical), University of Utah Department of Pathology. It can be performed on many automated analyzers, “so I would not describe it as a particularly esoteric test. Rather, it is not yet available on the extensive array of automated platforms that you would want at this point.” Ideally, it would be performed on combined chemistry-immunology instrument clusters so that cystatin C and creatinine can be performed at the same time without specimens having to be distributed among laboratory sections, he says. “But cystatin C is not yet in widespread use, and it adds significant cost.”
Roughly two percent of U.S. labs perform cystatin C in-house, says Greg Miller, PhD, professor of pathology, co-director of clinical chemistry, and director of pathology information systems, Virginia Commonwealth University, who was also a member of the NKF-ASN task force. Because it’s a send-out test for so many, coordinating results to perform the new eGFRcr-cys equation might present another challenge to labs, at least from an IT perspective, he says.
Clinicians have questions of their own. “If you bring it in-house, how do you then use it?” Dr. Powe asks. “Do you use it in everybody, or do you use it in a sequential strategy, applying it as a second test for individuals for whom you need more information for decision-making?”
For now, Dr. Hoenig says, her institution is not planning to run cystatin C as part of a regular metabolic panel; it’s a send-out test, though it’s likely to be brought in-house in the coming year. “We plan to offer cystatin C as a unique test that we can order if we have a question about kidney function and the creatinine. For example, perhaps a patient has very low muscle mass—like sarcopenia from cirrhosis—and we think the kidney function is worse than it appears. Or the opposite: A body builder whom we think has better kidney function than predicted with a creatinine-based equation. Cystatin C or creatinine-cystatin-C–based equations are particularly useful in these settings.”
Dr. Inker offers this example: A patient has eGFR around 50 but no albuminuria and no risk factors for disease. “eGFRcr-cys can help to determine if a patient has CKD or not,” she says.
In Dr. Hoenig’s opinion, for now, a cystatin C wouldn’t be necessary every time, but it could be measured once and then the creatinine followed from there.
The San Francisco VA already performs cystatin C as an in-house test, and Dr. Delgado sees the institution serving as a model for other VAs across the country as they adopt the second recommendation.
“One value does not make a diagnosis,” she says. “It’s a guide to help us discover, or make, the diagnosis, but it’s not the be-all and end-all. Which is why we rolled out two recommendations.”
For the 85 percent of Americans who do not have chronic kidney disease, “it’s going to be enough to make sure that their clinical needs are met,” says Dr. Delgado. But for the 15 percent who might have CKD, recommendation No. 2 will help.
For her part, Dr. Hoenig says, “I’m beginning to appreciate cystatin C more. I was using it before, but not that often. I’m pleased with how they [the New England Journal authors] also showed and did that additional work on cystatin C.”
The impact of the new equations remains to be studied—a point the task force makes in no uncertain terms. Much of the data the task force used to make projections “was based on simulations, not actual data and taking into account the behavior of physicians or patients,” Dr. Powe says.
As Dr. Powe’s experience shows, there was no telling for sure what might happen for institutions that acted before the task force’s recommendations came out, or even before the task force was formed. And as he noted in a viewpoint he wrote, titled “Black Kidney Function Matters: Use or Misuse of Race?” (JAMA. 2020;324[8]:737–738), the impact can run the gamut from administering/dosing medications to kidney donation and research study participation. And that’s the short list.
One of the biggest concerns Dr. Powe has heard from colleagues is what the new equation, once adopted, will mean for trending data. “What we really want to know in practice is if somebody’s kidney function is truly getting worse, and not getting worse because we changed the equation.
“That is an area that I think all institutions are going to have to deal with,” Dr. Powe continues, “and educate their workforce about how to interpret these changes. That’s one of the areas that everybody’s grappling with.”
The experience at Beth Israel Deaconess Medical Center might give a hint of what might transpire when eGFR equations do not have a Black race coefficient. BIDMC dropped the race coefficient from its eGFR reporting in 2017 and is widely considered the first institution to make such a move. Martha Pavlakis, MD, medical director of the BIDMC Transplant Institute, and Dr. Hoenig recently assessed the results.
Before the change, 26 percent of Black patients were listed for preemptive transplant, versus 70 percent of white patients. Post-change saw a steady increase in the percentage of Black patients listed before starting dialysis.
Drs. Hoenig and Pavlakis worried that Black patients would have a worse kidney function at the time of referral because without the multiplicative Black race modifier, the eGFR is lower. That was not the case. “The mean referral eGFR was higher for our Black patients after the change and on par with the non-Black patients. We were surprised,” Dr. Hoenig says.
Also, a small number of patients, nine, were listed for kidney transplant when the eGFR was ≤20 mL/min/1.73 m2 without the Black race coefficient, but the eGFR was too high if the Black race coefficient was still in use. These individuals “gained” an average of 475.9 days of wait time as a direct result of dropping the race coefficient when determining qualifying eGFR (Hoenig MP, et al. Clin Transplant. Published online ahead of print Oct. 3, 2021. https://doi.org/10.1111/ctr.14467).
Dropping the Black race modifier is just a small step, in their view, and significantly more change is needed to mitigate disparities in kidney disease.
The CAP has closely followed the work of the task force, says Dr. Genzen, who is chair of the CAP Clinical Chemistry Committee. Volunteers from that committee and the CAP Instrumentation Committee, as well as members with expertise in CKD and CAP leaders, drafted the CAP’s position statement supporting use of the new equations (https://bit.ly/CAP-eGFR).

Dr. Genzen
“Translating that into very simple guidance on what the laboratory should do is the next step,” he says. Dr. Miller says the aim is to provide an easy way to incorporate the equation into the lab information system and electronic health records so a standardized equation will be used in all laboratories. A special report (“National Kidney Foundation Laboratory Engagement Working Group Recommendations for Implementing the CKD-EPI 2021 Race-Free Equations for Estimated Glomerular Filtration Rate: Practical Guidance for Clinical Laboratories”) has been accepted by Clinical Chemistry and will be online soon.
ARUP plans to adopt the new equations and looks forward to publication of the report, Dr. Genzen says, so implementation across labs can be standardized. “We have faculty who are very engaged in this effort.”
“The actual implementation, once that clarity is shared, is not that complicated,” he adds. “It’s doable, and that’s why organizations like the CAP have taken a forward-thinking stance on emphasizing the importance of everyone doing this. Because we think through this standardization we can promote more equity in health care services across the country and internationally.”

Dr. Miller
Dr. Miller, whose institution has adopted the new creatinine equation, says: “Inside the lab, it’s primarily a matter of programming the new equation into the LIS. Outside the lab, it’s a matter of communicating to clinical colleagues that a change is coming and why it’s coming.”
Dr. Delgado says her institution is moving quickly to adopt the new creatinine equation. “There was a dedicated effort to hold off and wait,” she says. “Primarily they wanted to make sure we had a unified approach.”
Dr. Powe says San Francisco General also plans to adopt the new equation. But like many other institutions, it won’t be the first time they’ve made a change.
As Dr. Powe notes—and as he has experienced close up—“There’s not just one way to take race out of the estimation of GFR. But I think the reflex way that many institutions were doing it was to simply drop the race coefficient and assign African Americans the non-Black value, which is mostly the white value.” Others reported both values, or found ways to remove race from reporting results.
Dr. Miller points out that labs that changed their practices prior to the task force’s report are not following the recent recommendations. “This is a really important distinction,” he says. “The task force recommended replacing the old equation with the new equation refit without a race coefficient. That’s very different from removing race from the old equation. All of the coefficients in the new equation are different to reflect the refit without a race term.”
What if labs don’t adopt the new recommendations? The truth, says Dr. Delgado, is that even the current CKD-EPI equation is not in universal use.
It’s concerning, says Dr. Inker, that “some laboratories are still using outdated methods, including MDRD or methods that don’t use standardized reference methods. So this is an opportunity to have uniformity in GFR reporting across the country.”
Dr. Powe speaks in measured tones, eschewing the role of evangelist or cheerleader for the recommendations. “I think—and this is my personal belief—that every institution needs to look at what we came up with, and the rationale, and the data, and whether they believe what we did is a sound approach. And decide, just like they did before, whether it’s a better approach.” But if institutions do endorse it, he adds, “our hope is that they implement it quickly.”
Dr. Delgado would like to see the recommendation sink in broadly and illuminate where challenges remain. “I want this work to stand,” she says. “And I want this work to reflect that this is not a perfect test. There’s imperfection in the estimate, and we should be aware of that and teach our patients that.”
Dr. Powe says the new approach does address the concerns he had about quick fixes—he calls it sound and evidence-based, and says it is unlikely to cause harm.
“GFR is not an easy concept,” he says—medical students and physicians alike struggle with the nuances. Trying to grasp how the earlier equations evolved only compounds the difficulties. “How did we get ourselves to this place?” Dr. Powe asks. “We were having kind of these knee-jerk responses to jettison the coefficient. You could just do it overnight. And some did. But it may have caused more harm than good.”
This was Dr. Delgado’s first time leading a task force. “We had people who truly had varying opinions on how to come up with a solution,” she says, given that their backgrounds ranged from patient advocates to those with research expertise in estimating equations, and from experts in laboratory medicine to pharmacotherapy to those with expertise in identifying health care disparities.
With so many voices in the mix, “I thought it was quite amazing that we all had the same unity and desire to come up with an approach that did not disadvantage anyone,” Dr. Delgado continues. She credits in part the evidence and values statement table that appeared in the interim report. Even with that in place, however, “Getting to the end was a challenge,” she says.
Dr. Powe too credits the guiding hand of the evidence and values statement. “That helped us to see a path through all the different alternatives. That was pivotal, and it allowed us to go through in a thoughtful manner and select the best approach we could find at this point in time.”
If that sounds temporary, well, it is. As Dr. Inker notes, echoing recommendation No. 3, “There is much more work to be done. Our hard work is just beginning, and that can’t be forgotten.”
Adds Dr. Powe: “I hope that this stimulates conversation of what the main issue is.” African Americans are more likely to develop kidney disease, and at an earlier age, than other groups, disparities that have been documented for 40 years, he says.
“It’s important to recognize that disparities on the transplant waitlist and for nephrology referral were there more than a decade before race was even used in the [eGFR] equation,” he says. “A lot of people don’t realize that—that the equation didn’t cause those disparities, although that has been a lot of the rhetoric.”
“What does that tell you?” he continues. “It tells you there are other reasons—and that’s where I’ve done a lot of work in my career, trying to understand the genuine drivers of these disparities, and keep our sights on those. And not simply believe that the change in the equation is going to wipe out those disparities.”
Solving problems that have been decades or more in the making won’t happen overnight, Dr. Powe says. “There are many other things we need to do if we’re thinking we’re going to achieve health equity. And they’re far greater than changing the equation.”
Karen Titus is CAP TODAY contributing editor and co-managing editor.