William Check, PhD
June 2016—Laboratories that use the HIV testing algorithm the CDC recommended in 2014 report shorter turnaround times for those with detectable antibodies. And among state and local public health laboratories that responded to a 2015 survey, more than half report having implemented the algorithm. This was just some of the information presented in March at the annual HIV Diagnostics Conference, where speakers, a handful of whom spoke with CAP TODAY since, shared data on the use and efficacy of the algorithm.
“From a recent Association of Public Health Laboratories survey [reported at the conference] we know that the algorithm has been implemented in approximately 55 percent of state and local public health labs that responded,” says Michele Owen, PhD, conference co-chair and the CDC’s associate director for laboratory science, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. “We also have limited data indicating some labs are using parts of the new algorithm.”
“So far the biggest success associated with implementation of the algorithm,” she says, “is the drastic improvement in result turnaround times for HIV-infected individuals who have detectable antibodies—those who are positive at the second step of the algorithm.”

Dr. Pentella
Michael Pentella, PhD, D(ABMM), director of the Massachusetts State Public Health Laboratory, presented data from the APHL survey. “In 2009, before the [2014 CDC] algorithm was introduced, about 48 of 61 public health laboratories that responded said they were using the Western blot test,” Dr. Pentella tells CAP TODAY. The 2014 algorithm recommended that the Western blot be replaced with an assay that differentiates antibodies to HIV-1 and HIV-2, such as the Bio-Rad Multispot rapid test, which turns positive a week earlier after infection than the Western blot. By 2015, the number of laboratories using the Western blot had dropped to 10 of 74 responding labs that were doing HIV testing at the time of the survey. “We don’t know why those 10 are still doing it,” Dr. Pentella says.
Thomas Alexander, PhD, D(ABMLI), recalls his laboratory having a problem with the initial fourth-generation combination assay in 2010, before the CDC’s algorithm became available. They had a sample that was positive in the combination assay but negative on the confirming assay. “The person exposed turned out to be a low positive,” says Dr. Alexander, who is an immunologist in the special procedures laboratory in the Department of Pathology and Laboratory Medicine Group, Summa Health System, Akron, Ohio. “We had to wait for three tests to find out that it was falsely negative. It was more expensive, it took more time, and the infection control department wasn’t wild about it.”

Dr. Alexander
The newest type of combination test, which differentiates immediately among positivity to HIV-1 and HIV-2 antibody and p24 antigen, can prevent these types of situations, he says. “With antigen-positive, antibody-negative primary infection you only get a single result with [fourth-generation assays]. You have to do an antigen confirmation test. The advantage of the so-called fifth-generation tests is you get a separate result [for antibody and antigen]. You can confirm with that one test.” Dr. Alexander acknowledges that these cases of acute infection are uncommon in his patient population. He estimates that he has had fewer than 10 specimens that required that third level of testing. “It’s very rare that it happens, but it does.”
In practice, it appears that fourth- and fifth-generation combination assays are similarly effective in detecting HIV infection, according to data reported by Teal Clocksin, MS, technical specialist at TriCore Reference Laboratories in Albuquerque, NM. “What’s important for people to understand is that the lab-based HIV combination assays from all three of these manufacturers are quality assays,” Clocksin says of the Abbott, Siemens, and Bio-Rad assays. “The real challenge is getting laboratories to use them.” There are sensitivity differences among them, he adds, “but those differences are minor for our population.”
Additional positive data on the fourth-generation algorithm came from Christopher Pilcher, MD, associate professor of medicine in the HIV/AIDS Division, University of California, San Francisco. In his talk, Dr. Pilcher reported that implementing the algorithm for high-throughput testing every two hours was feasible in a large hospital-based clinical laboratory. He and his colleagues found that the number of infections classified correctly without send-out testing was greater compared with their previous testing procedure.
“The new algorithm is designed to allow quicker and more accurate resolution of infection status and did actually get slightly faster results to patients overall,” he tells CAP TODAY. However, the type of results available in the first day or two led to confusion in certain cases, he says, because the positive predictive value of an initial positive result is low in low-prevalence screening populations. Clinicians and patients need to understand the meaning of these early results.
Laboratory testing for HIV has progressed over three decades from simple tests that detected only antibody against HIV type 1 to combination tests that simultaneously detect antibody to types 1 and 2 as well as p24 antigen, which appears in the blood weeks earlier than antibody. Molecular tests detect HIV nucleic acid, the first viral product to rise in the patient’s blood, within days of initial exposure/infection and a week or so ahead of fourth-generation combination assays.
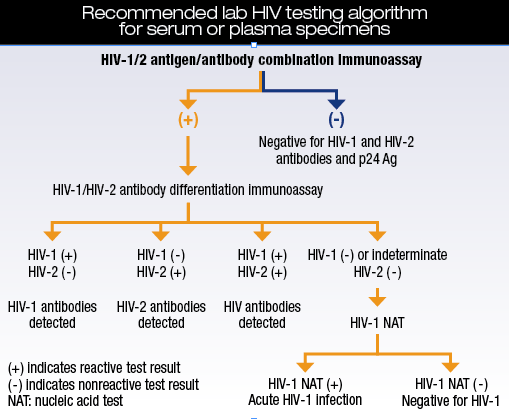
Source: CDC/APHL
Combination assays that simultaneously detect antibodies to HIV-1 and HIV-2 and p24 antigen are considered fourth-generation tests. Most recently, the FDA approved a test for aiding in HIV diagnosis that some consider fifth generation: It provides separate results for HIV-1 and HIV-2 antibodies and HIV p24 antigen. The confirmatory test algorithm has not been determined, but it may be simpler than for fourth-generation tests.
Optimal use of fourth-generation assays as the first step in screening requires a subsequent HIV-1/2 differentiation step for positive samples and, in some cases, a nucleic acid test. This constitutes the fourth-generation algorithm. Data presented at the conference showed that this algorithm can reduce turnaround time in some cases and may reduce cost, says Dr. Owen of the CDC. “Data from the City of Milwaukee Health Department’s Public Health Lab showed an average turnaround time for antibody-positive individuals to be 23 hours and 47 minutes with the 2014 algorithm compared to one to two weeks before implementation of the algorithm.” Mayo Medical Laboratories showed a decrease of 10 hours in TAT for antibody-positive individuals.
In addition, the Mayo laboratory showed that the new algorithm was 45 percent less costly for them to perform for antibody-positive specimens compared with the previous algorithm that used Western blot, Dr. Owen says.
Some of the laboratories that have not adopted the algorithm cite the expense, Dr. Pentella says. “From our experiences, we know that it does take a lot to make the change, to do the necessary validations you need to do, and to educate the providers on what you are now going to be reporting to them and why.”
Dr. Pentella adopted a fourth-generation test when he was associate director at Iowa’s public health laboratory in 2012. “We invested time to look at new automated instrumentation. We had to validate the test. We had to write new procedures, train our staff, and then we had to train our facilities that collected samples and sent them to us. There was a lot of education that had to be done. We had to find funds to do that.”
The effort and expense were justified, in his estimation. “We thought we needed to do it to offer a better assay and to be able to detect people in the acute phase of infection,” when they are most likely to transmit. “And it’s also for the patient,” to get them into treatment faster.
“That’s what we’d like clinical laboratories to think about, too,” Dr. Pentella adds. “Many clinical labs use a fourth-generation antigen-antibody assay. If you go to a Western blot as your next test, you could have a false-negative on the Western blot. If you don’t look carefully at when a test is going to be a positive, you could miss a truly infected individual.” CAP Surveys also continue to see use of the Western blot.
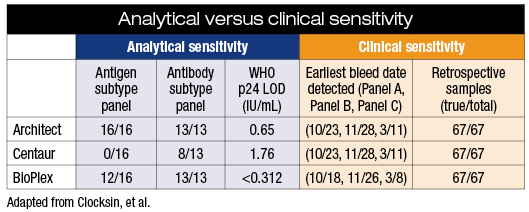
Clocksin and colleagues at TriCore have been doing HIV screening on an automated track using the fourth-generation algorithm. “We’re able to turn around an HIV screening result generally within 12 hours,” he says. Working with the New Mexico Department of Health state laboratory, they compared the sensitivity of the three combination tests approved for HIV diagnosis: Abbott Architect HIV Ag/Ab Combo, Bio-Rad BioPlex 2200 HIV Ag-Ab, and Siemens Advia Centaur HIV Ag/Ab Combo (CHIV).
All three assays performed well on an HIV subtype antibody sensitivity challenge (see “Analytical versus clinical sensitivity,” page 17). On a comparable subtype antigen sensitivity challenge, Architect and BioPlex did well but Centaur missed all 16 samples. Finally, using 67 retrospective patient samples from their specimen repository, half reactive and half nonreactive, all three assays were 100 percent accurate.

Clocksin
“It’s important to consider that in the areas where the Centaur appeared to perform worse, those were contrived samples,” Clocksin explains. “Those were samples diluted down to assay-discriminating concentrations. So if you look at the actual, native patient samples, there were pretty minimal differences between the three assays.”
They decided to go with the Centaur. “We felt it performed equivalently when it comes to actual clinical patient samples. Also, because it can connect to the track, it has far better turnaround time, and that makes a big difference to our clients.” The BioPlex cannot connect to their automated track.
The fifth-generation BioPlex assay is not FDA approved as a confirmation assay, Clocksin notes. “If you have a positive sample, you still have to move on to the confirmation test.” Of the BioPlex’s ability to discriminate HIV-1 from -2, Clocksin says, “In the U.S., where HIV-1 is so much more prevalent, I’d question the advantage [of the BioPlex HIV-1 and HIV-2 differentiation ability].”
Because the Centaur and Architect were less sensitive in pre-seroconversion samples, there is a slight possibility an acute infection could be missed. However, in a low-prevalance population this is extremely unlikely, Clocksin says.
Dr. Pilcher and colleagues evaluated the “real-world performance” of the fourth-generation HIV algorithm in medical settings. Over the past 10 years physicians at San Francisco General Hospital (recently renamed Zuckerberg San Francisco General Hospital and Trauma Center) have been aggressive in expanding the proportion of patients in all medical settings who are HIV tested, Dr. Pilcher says. “We have especially focused on the emergency department, where most patients are admitted, as well as expanding routine testing in clinics and urgent care settings. One of the key points of this study was to see how accurately and quickly the urgent testing algorithm was resolving infection status and how quickly we can return accurate results that clinicians can act on.”
The program not only does routine HIV screening as part of primary medical care and public health screening, he says, but also documents HIV status upon entry to the hospital—the main point of his talk at the HIV conference. “By far most positives come from patients who are already known positive,” Dr. Pilcher says. What clinicians want to know, however, is how accurate a test result will be when the patient’s HIV status is unknown. A test might detect 50 true positives and turn up five false-positives, which may appear to be good. But if the 50 true positive patients were already known, then among those whose status is unknown there are no true positives and five false-positives. Not so good.
Until September 2015 the hospital laboratory was using a rapid test followed by immunofluorescence confirmation. They adopted the fourth-generation algorithm that September, and time to completion of the algorithm was substantially less than the time to a confirmed result with the previous strategy—21 versus 71 hours. The need for send-out testing was greatly reduced also. Indeterminate results after a confirmatory antibody test went from 40 with immunofluorescence to none with Multispot. Specificity was unchanged at 99.7 percent.
In a second evaluation, results at the hospital were compared with those from men presenting, because of high-risk behavior, for voluntary testing at HIV clinic testing sites, known as STOP sites. Specificity was very high in both groups. However, positive predictive value after an initial positive result differed greatly: 0.44 at the hospital versus 0.93 at the STOP sites.
That’s because the prevalence of new cases was 1.55 percent at the STOP sites, but only 0.23 percent at the hospital. The number of false-positive cases was substantial: Of 27 initially positive specimens in the hospital cohort, 14 turned out to be HIV-negative.
 Moreover, no acute cases were found in the hospital population, so the positive predictive value of an initial positive followed by a negative Multispot was zero, whereas at the STOP sites it was 0.62. “This result was shocking to us,” Dr. Pilcher says. To sort that out, an RNA test is needed, so resolution can take several days. While waiting for the results of the nucleic acid amplification test, or NAAT, some clinicians and patients decided to initiate antiretroviral therapy, only to discontinue it when full results were known. Handling disclosure to partners is difficult in this situation. “Be very cautious in counseling patients and starting on therapy,” Dr. Pilcher advises. “Think about waiting for completion of the full algorithm.”
Moreover, no acute cases were found in the hospital population, so the positive predictive value of an initial positive followed by a negative Multispot was zero, whereas at the STOP sites it was 0.62. “This result was shocking to us,” Dr. Pilcher says. To sort that out, an RNA test is needed, so resolution can take several days. While waiting for the results of the nucleic acid amplification test, or NAAT, some clinicians and patients decided to initiate antiretroviral therapy, only to discontinue it when full results were known. Handling disclosure to partners is difficult in this situation. “Be very cautious in counseling patients and starting on therapy,” Dr. Pilcher advises. “Think about waiting for completion of the full algorithm.”
The CDC’s Dr. Owen says one of the largest hurdles to implementing or achieving the maximum benefit from the 2014 algorithm is the lack of easily implemented and affordable FDA-approved diagnostic nucleic acid tests. Data presented at the conference suggest that time to NAAT results can range from five days to two weeks after a specimen is submitted for testing, greatly increasing turnaround time for those who are acutely infected.
“We know there are rapid nucleic acid tests currently in the pipeline for submission to FDA that have the potential to improve the turnaround time for nucleic acid test results,” Dr. Owen says. “However, these are likely a year or so out. An alternative approach would be for labs to validate off-label uses of FDA-approved viral load assays, and we know this has been done in some locations,” such as the San Francisco Department of Public Health laboratory and the Harris Health System in Texas. “However, the off-label validation of a test also has significant costs, including the staff time needed to gather the required data for validation.”
An optimal solution, she says, would be for manufacturers of current FDA viral load tests to seek and receive a diagnostic claim for their test. To date, however, the manufacturers have indicated the cost of seeking the additional claim is prohibitive under the current FDA requirements.
Even so, approval of rapid viral load tests is the answer, in Dr. Pilcher’s view. “It is extremely important to pressure those companies to get their tests approved as quickly as possible for diagnostic use. Having a rapid viral load result would enable laboratories to confirm a patient’s status within minutes rather than days,” he says.
At TriCore, in-house validation is underway. “We’re working on validating a viral load assay as confirmatory testing,” Clocksin says. “Right now, we run the Roche HIV-1 quantitative test [Cobas AmpliPrep/Cobas TaqMan HIV-1 Test, v2.0], and we’re currently validating some additional testing. The goal for a big lab like us is being able to run through the entire algorithm without having to go back and ask a patient for an additional sample collection. It is difficult to complete the NAAT step of the algorithm once you start asking patients to come back for additional blood draws.”
Not every laboratory can do this. “In my situation, in a relatively low-risk area, I’m never going to have enough primary antibody-negative infections to validate and to meet the CAP’s expectations,” says Dr. Alexander of Akron’s Summa Health System. “A Roche representative told me they are looking to obtain a qualitative result option for diagnostic use in their next-generation of HIV nucleic acid test, in addition to the quantitative monitoring approval.” Whether and when that happens is an open question.
The other test talked about at length at the conference was the fifth-generation assay. “The introduction of the BioPlex HIV Ag-Ab test has the potential to change the recommended lab algorithm,” Dr. Owen tells CAP TODAY. “It is feasible that the ability to differentiate HIV antigen [p24] reactivity from antibody reactivity could lead to a change in which nucleic acid testing is recommended after an antigen only positive result. However,” she cautions, “we do not have sufficient data to make a formal recommendation at this time.”
Dr. Owen said the CDC has looked into when the BioPlex assay becomes reactive compared with other antigen-antibody tests. “Preliminary data suggest it performs comparably with other lab-based tests that require serum or plasma,” she says.
At the Massachusetts State Public Health laboratory, Dr. Pentella and colleagues are considering the fifth-generation Bio-Rad BioPlex. “We also test hepatitis C virus on the same platform as HIV. The reason I’m not so enthusiastic is because the product for the fifth-generation assay doesn’t have HCV. Both of these diseases go hand in hand, and at times that’s very important. They have the same risk factors.”
Dr. Pilcher doesn’t see his laboratory introducing the fifth-generation test anytime soon. “It wouldn’t solve any problems,” he says. “Without the capacity to do a rapid RNA test you will have exactly the same problem I presented. With some cases we encountered it might have been easier to sort out false-positive from true positive results. But those are rare. The fundamental problem is the wait for viral load to come back, which is not addressed by the fifth-generation test.”
He calls the test “just a different format of the fourth-generation test.”
Dr. Alexander says he is interested in evaluating the fifth-generation test versus the fourth-generation tests. “The kind of end result of the meeting was, yeah, we need to do something about this,” he says. “Exactly what’s going to be done isn’t clear. In this situation, if you’re doing the Alere or the BioPlex method and you have someone who’s antigen-positive but antibody-negative, then why do a Geenius test on that? There’s no antibody positivity, so why waste the time or the expense of doing that additional test? You should go right to the molecular confirmation.”
[hr]
William Check is a writer in Ft. Lauderdale, Fla.