Sherrie Rice
June 2022—What to look for in serous fluid cytology is what Eva M. Wojcik, MD, of Loyola University in Chicago, and Xiaoyin “Sara” Jiang, MD, of Duke Health, set forth in their CAP21 session last year.
“Mesothelial cells are difficult for so many people, and they’re difficult for me,” Dr. Jiang said, “because they’re present in nonmalignant cytology samples from the serous fluid cavities, and a lot of patients who have infections or chronic heart failure or other conditions that involve fluid cavities will have reactive mesothelial cells. And reactive mesothelial cells can be present as single cells, clusters, balls, or sheets.”
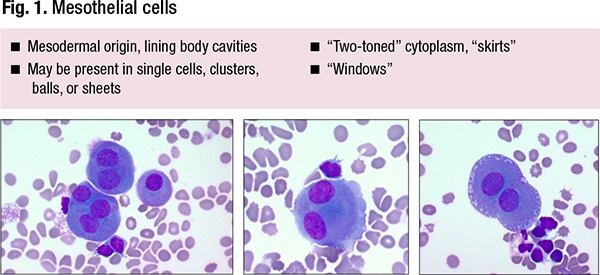
The classic features can be seen in Fig. 1, among them “windows,” or tiny gaps between the adjacent cells. “I think they’re much more like alleys than windows,” said Dr. Jiang, chief of the Duke head and neck pathology service. They often have so-called skirts—“lacy” cytoplasmic borders. They can be multinucleated and enlarged.
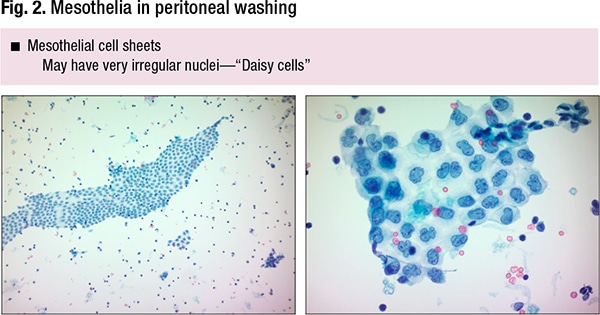
In peritoneal washings they can look very different, she said—mesothelial cell sheets (Fig. 2). “They tend to be relatively bland-looking from low power, well organized, almost like a honeycomb,” she said, but sometimes due to processing artifact they can have very irregular nuclei with lobulated contours, “so-called ‘Daisy’ cells. If you’re not aware this is something that can normally happen in mesothelial cells, it is potentially a pitfall for someone new to cytology.”
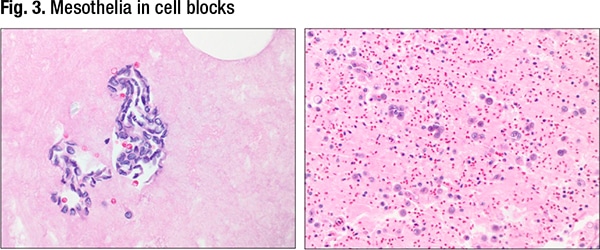
In Fig. 3 are mesothelia in cell blocks.
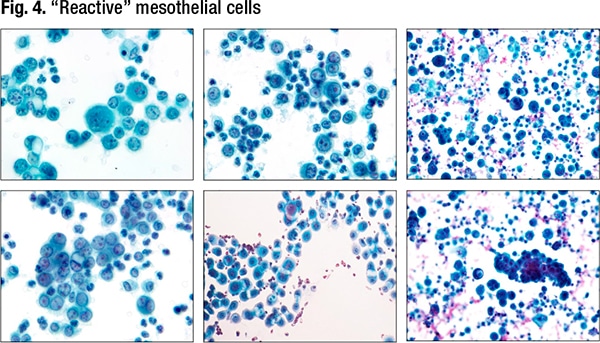
When reactive, mesothelial cells can have cytoplasmic vacuoles, “which makes you wonder: Are these histiocytes, is it adenocarcinoma? They could be multinucleated, have nuclear contour irregularities, coarse chromatin, and occasionally even mitoses,” Dr. Jiang said. Reactive mesothelial cells can be difficult to distinguish from a second population of tumor cells. (Fig. 4).
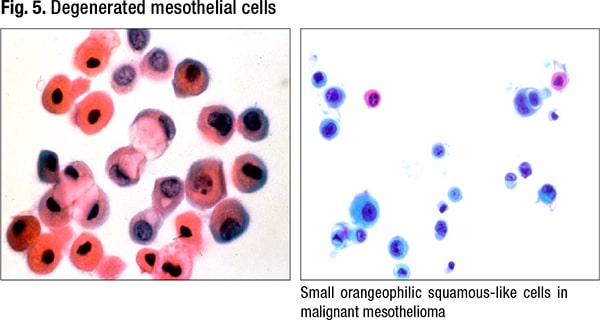
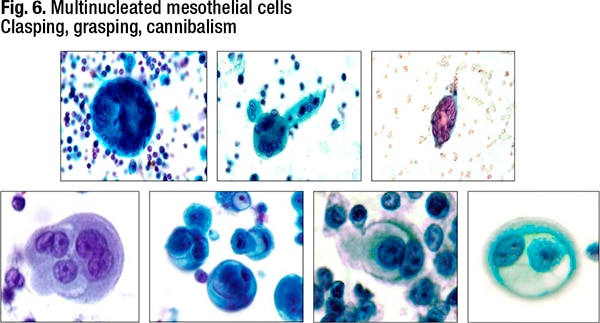
Degenerative changes can also be challenging, she said, noting that some degenerated mesothelial cells can look squamous-like (Fig. 5). “And you can get these clasping, grasping, cannibalism appearances as well,” Dr. Jiang said (Fig. 6). In reactive mesothelial cells, the feature of cannibalism creates worry about cannibalism in adenocarcinoma, and these features can be seen in both benign and malignant mesothelial processes. “When you see the giant cells with multiple nuclei, it can be a little difficult to distinguish histiocytes versus mesothelial cells.”
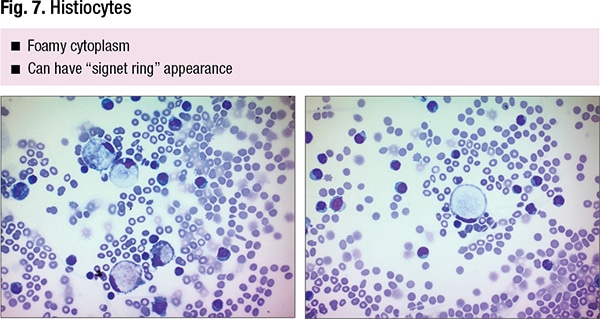
Dr. Wojcik, chair of Loyola’s Department of Pathology and Laboratory Medicine, agrees and says histiocytes are “underrepresented in our textbooks.” Signet ring, vacuoles in mesothelial cells—“that’s exactly what you’re going to see in histiocytes also,” she said. In Fig. 7 is a “perfect signet ring, and whoever sees signet rings will get concerned that we’re missing signet ring carcinoma because they can look exactly the same.”
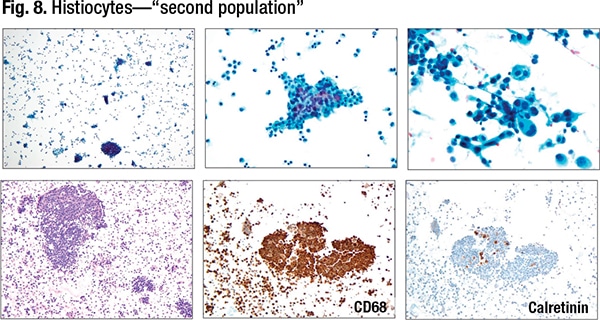
Histiocytes can look very atypical, she said, and they can also be numerous. Engraved in the mind of any cytopathologist is “two populations in fluids equal malignancy.” Histiocytes can cluster too. “We’re told over and over: Histiocytes usually are arranged singly. But they can cluster within the fluids, even in the cell block. And on top of that they can appear atypical.” (Fig. 8).
Since the two-cell population wouldn’t prompt thoughts of histiocytes, Dr. Wojcik said, an immunohistochemical panel would be done to differentiate epithelial from mesothelial phenotype. “Both of them are going to be negative, and potentially we are on the spiral into some very weird differential diagnosis.” For that reason, she often uses CD68—“the eye-openers,” as she puts it—to identify the histiocytes.
“Most of us say, okay, this is mesothelial, it’s benign. It’s negative, no issue. But once in a while, especially when they cluster, when they have atypia, that could be a challenge, so include CD68 in your panel.”
Dr. Jiang’s experience is the same: “Everything [then] lights up with CD68,” she said. “It’s amazing. They’re so tricky—histiocytes and mesothelial cells. They’re the bane of our existence sometimes.”
For malignancies, the general principles begin with the following, Dr. Jiang said: “Look for the second population of tumor or, if you have histiocytes, it might be a third population.” In addition, large cell clusters are concerning—“always something worrisome and depending on whether it has knobby or ‘mulberry’ borders,” she said, generally indicating mesothelioma, or smooth/community borders, often indicating metastatic carcinoma.
If a cell block is used, retraction around metastatic clusters can be a helpful finding. And when looking at fluids, she said, this artifact is much more common in metastasis than primaries. “For the most part,” Dr. Jiang said, “patients who have metastatic effusions usually already have a clinical history of a known malignancy. Rarely will an effusion be the first sign of malignancy.”
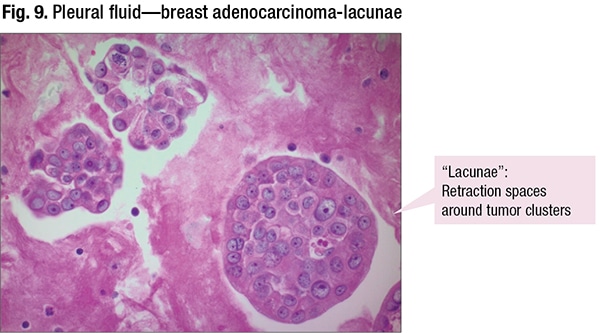
Adenocarcinoma is the most common cause of malignant effusion. Seen in the cytology will be increased N/C ratio, irregular nuclear membranes, large nucleoli, secretory vacuoles, and 3D clusters with smooth/community borders or cannonballs. The retraction artifact is seen in Fig. 9. “There’s a retraction space between the tumor cells and the fibrin in the cell block. It’s a little white space, a little lake that these are sitting in. And the retraction artifact is a soft sign that you’re dealing with malignancy and it can help as you build your diagnoses,” Dr. Jiang said, “but like anything in life and medicine, this is not 100 percent.”
Cannonballs are most commonly seen in breast cancer, but can also be seen in other cancers such as lung and in particular in ovarian carcinoma, Dr. Wojcik said. “Cannonballs, cell balls, morulas—these are the classic patterns when we have the three-dimensional structure” (Fig. 10). When talking especially about adenocarcinoma, they are hollow inside, she said. “You’re going to see this especially in the cell blocks but even in smears. And that can be helpful when you are differentiating with mesotheliomas, which rarely will have a collagenous core inside.”
Though smooth borders are common in adenocarcinoma, Dr. Wojcik notes the “beautiful knobby borders” that can be seen in adenocarcinoma (Fig. 10, top image). “In mesothelioma you will see very smooth borders as well,” she said. “That’s why we need everything, not only one feature but all the features to be put together, and in many cases immunohistochemistry.”
Other patterns: single cells, single cell files, bizarre or giant cells, clear cells, and 3D clusters. The single cells are the “tough ones,” Dr. Wojcik said. “Especially the mesothelial pattern.”

In Fig. 11 is the “mesothelial” pattern, which Dr. Wojcik describes as “really scary.” When the patient has a history of breast cancer, she said, “we have to be very careful not to miss it, and the scary part is, when you’re going to do immuno and they’re all positive—the first thing you think is, How many did I miss before? Because without that history, it would be very difficult to start even thinking” about breast carcinoma.
One clue, she said, is a uniform pattern, single cells, with almost a “feeling of clonality.” The cells are similar. “Look under higher power at those nuclear membranes—some of those cells have very irregular nuclear membranes,” while mesothelial cells usually do not to that extent. Another clue: always look for targetoid mucin.
In Fig. 12 is a single cell arrangement of lobular carcinoma, where another clue is targetoid mucin. In Fig. 13 is pseudomyxoma peritonei. “The problem with this one,” Dr. Wojcik said, “is the nuclear atypia is minimal. These are low-grade, slow-growing tumors, and the issue is thick mucinous effusion. It’s difficult to clear and that is clinically the biggest issue.” The mucoid background is most important.
In Fig. 14 is small cell carcinoma. “This one can be tough because they usually are not very cellular,” she said. The small, tight clusters of cells are easy to overlook. “They’re not much bigger than the lymphocytes, and only when you have them side by side can you see tumor cells are larger.” Always look for apoptosis, she advises: “You will see apoptosis even on the cytology specimen.”
Hematopoietic malignancies are seen often. Of the lymphomas, CLL can be tricky, Dr. Wojcik said, because they look almost mature. One pitfall to remember: “If you have a patient with CLL, you’re going to zoom in to those lymphoid cells and potentially find some cells that can look abnormal, but keep in mind that potentially those cells are coming from peripheral blood.” Plasma cell neoplasms can also be seen in the fluids.
In Fig. 15 is an example of CLL in which a monotonous population and the characteristic coarse “soccer ball” chromatin are seen, Dr. Wojcik notes. “They are a little larger than regular mature lymphocytes, but it would be difficult to evaluate in fluids. A lot of lymphocytes—make sure it goes for flow.”
In her mesothelial versus adenocarcinoma immunohistochemistry panel, Dr. Jiang likes to use D2-40, WT-1, and calretinin for mesothelioma, “which for the most part are negative in adenocarcinomas,” she said, though “there can be overlap where WT-1 and D2-40 can be positive in ovarian carcinomas.” Her markers for adenocarcinoma are Ber-EP4, MOC-31, and more recently claudin-4, which tend to be positive in adenocarcinomas and negative in mesothelial cells. If there is a known malignancy, or the patient has a mass somewhere, she adds TTF-1 (lung adenocarcinoma and thyroid), p63 (SCC, urothelial, others), and Pax-8 (RCC, ovarian, thyroid). WT-1 and D2-40 are positive in serous carcinomas of the ovary, she noted. “There’s no one-size-fits-all approach,” Dr. Jiang said. She advises extra caution when doing a GATA3 because “very rarely, mesothelial cells can be positive for that.”
Cell blocks can present challenges, too, Dr. Jiang said, noting that she often sees rare, atypical cells on the ThinPrep or cytospin. “And with cell block, you may not see a really distinct population of big cannonballs,” she said. “You may just have one or two single cells that are atypical. If you’re using immunos in that context, be really cautious about making the definitive diagnosis of malignancy when dealing with single cells.” Some cases surprise, she said. “You do the GATA3 or the ER and everything lights up. But often it’s rare single cells that maybe have a little TTF or a little GATA3 in the cell block.”
“That’s why we still have the indeterminate diagnosis,” Dr. Wojcik said. “This is for cases where we try to work it out, and despite our efforts, we cannot make the diagnosis straight. However, we cannot ignore them.” If the patient reaccumulated fluid quickly, that’s a red flag to look at this much more seriously, she said.
Sometimes, Dr. Jiang said, it’s simply a case of the cytopathologist catching the tumor early on, when there is little tumor burden. “A couple of weeks later, they recollect, and then it becomes much more obvious. We’re catching tumors at different points in their biology.”
“What if we’re left with something that looks mesothelial and stains mesothelial and then looks malignant?” Dr. Jiang asked.
“We make a diagnosis of mesothelioma,” Dr. Wojcik replied, noting it’s the subject of controversy, especially in cytology. Malignant mesothelioma is a serious diagnosis, but there is an opportunity for management and patients have a chance for an extended survival if diagnosed early, she said.
All of the characteristic features of mesothelial cells will be seen in mesothelioma: grasping, clasping, cannibalism, prominent nucleoli, multinucleation, and the others. One way to differentiate: There will be more of those features and all will be larger, Dr. Wojcik said (Fig. 16).
“When you see huge macronucleoli, think potentially about mesothelioma,” she said (Fig. 17). Near perfect symmetry and vacuolization can also be seen.
Nuclear atypia is where the controversy lies, she said (Fig. 18). It’s not classically striking, “especially when you see the uniformity of cells.”
Another clue: small, orangeophilic squamous-like cells (Fig. 19). “This is where we are going to zoom and think, Is this a squamous cell carcinoma? But they don’t have enough atypia for it to be a squamous cell carcinoma.” The diagnosis can’t be made based on these cells, she noted, “but when you see them, think, Could this be a case of malignant mesothelioma?”
Fig. 20 is “mesothelioma in cytology in real life,” she said, noting they were the most abnormal case she could find, confirmed by IHC as mesothelial. In cases like that, she said, “there’s no way we can make the diagnosis on cytology even if we are aware that the concomitant surgical specimen was diagnosed as mesothelioma.”
How to make the diagnosis? First, establish the mesothelial phenotype. (Only the epitheloid type of mesothelioma, or a mixed type with a significant epithelial component, will shed the cells that can be evaluated, she noted.) The next step is to establish malignancy by FISH (deletion of the 9p21 region/loss of p16 [CDKN2A]). Other markers: BRCA1-associated protein 1 (BAP1) mutations (loss) and methylthioadenosine phosphorylase (MTAP) 9p21.3-related protein (loss). “It’s very important to have an internal control,” she added.
Thus, cytology specimens can be used but the diagnosis shouldn’t be made on morphology alone. Malignant mesothelioma in situ “is real,” she said, noting the consensus of experts. Dr. Wojcik’s approach to malignant mesothelioma on cytology is what she calls “another triple test” (Fig. 21). In a case with a characteristic morphology and an encasing mass on CT, for example, “I don’t need cytology. I know it’s malignant mesothelioma,” she said, adding that it can be confirmed by loss of BAP1. For legal purposes, she said, if there is certainty based on morphology, clinical radiographic presentation, and markers, it’s better for all to make the definitive diagnosis. Use of “consistent with” is likely to raise questions.





If the clinical presentation suggests malignant mesothelioma and there is a characteristic morphology but the markers are inconclusive, the diagnosis is “malignant cells most consistent with malignant mesothelioma.” If something doesn’t make sense, she said, “we have to leave ourselves room.”





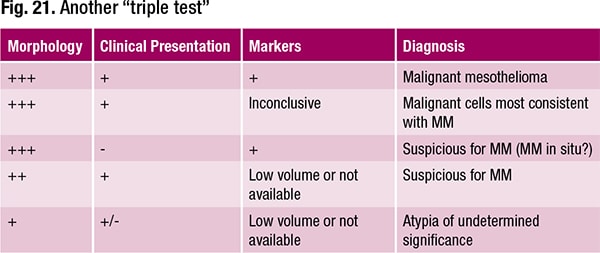
If the clinical exam turns up nothing but the markers are consistent with malignant mesothelioma along with a positive control of benign mesothelial cells, “you cannot ignore it,” she said. “You can’t let it go. You have to tell clinicians they have to find something. This is potentially mesothelioma in situ.”
If there’s a mass but an insufficient or unavailable specimen for additional studies to confirm malignancy, it’s atypia of undetermined significance and there must be follow-up.
“Whatever you do, avoid extremes,” she advises. “All the pieces in your puzzle—morphology, clinical/radiographic findings, and, most importantly, adjuvant tests—have to line up to make a diagnosis of malignant mesothelioma in cytology.”
Sherrie Rice is editor of CAP TODAY.