Anne Paxton
July 2023—When Butler Health System in early 2020 installed the Beckman Coulter DxA 5000 automation line, its hospitals were among the first in the country to do so. At the same time, Butler went live with the DxH 900 hematology analyzer. The hope was that the new automation would ease the laboratory’s labor shortage, says Robert Patterson, MD, director of pathology and laboratory medicine for the Butler system, based in Butler, Pa., and as of this year part of the Independence Health System.
By 2022, the new automation was helping in another way: by reducing the time to sepsis diagnosis and patient management.
The Food and Drug Administration in 2019 cleared the monocyte distribution width sepsis biomarker as a reportable parameter included in the leukocyte five-part differential analysis. After using the MDW for 10 months, starting last year, Dr. Patterson can report that time to diagnosis and appropriate therapy is shorter. He shared his lab’s findings this spring at the Executive War College and in a recent interview with CAP TODAY.
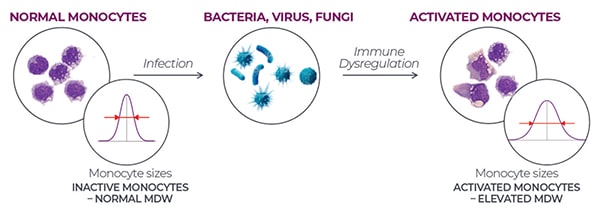
MDW is the calculated standard deviation of the measured monocyte volumes. A normal MDW would be the result if monocyte sizes indicated inactive monocytes. But when an infection—whether bacterial, viral, or fungal—leads to immune dysregulation that decreases the relative number of monocytes, there is a subsequent increase in the variability of monocyte sizes and an elevated MDW, which may indicate sepsis is a possibility.
One of the most important changes in diagnosing sepsis in the past 10 years has been the evolution of criteria for clinically suspecting that a bacteremia patient has sepsis, Dr. Patterson says. “On the laboratory side, there have been molecular advancements with some of these phenotypic panels. We’re able to detect a certain group of organisms, some of which also have resistance genes attached to them. And those can be helpful. But you still need to have an antibiotic.” And determining the right one is not simple. “The reason is that in the Gram-negative organisms, there are so many possible mutations. And as soon as a manufacturer or vendor patents one certain panel with a set of genes on it, it’s obsolete, so it isn’t that helpful.”

Dr. Patterson
Dr. Patterson and Butler’s emergency department head and infectious diseases director joined as a cross-functional team to consider how they might be able to address the gap by using MDW—but carefully. “MDW is elevated in bacterial sepsis. It also can be elevated in viral sepsis [viremia] or with fungal systemic infections [fungemia], as well as with some noninfectious causes such as pregnancy. Those are other possibilities,” Dr. Patterson notes. The team created an algorithm using both MDW and WBC, and they decided to include abnormal WBC (high or low), rather than just an elevated WBC, as an additional criterion for concluding there is a substantial risk of sepsis. This was done because there are outliers—the elderly in particular. “When they get sepsis, their WBC often goes down,” he says.
Once an MDW hits a value of 20, the laboratory sends a blood sample to its T2 Biosystems instrument, which has a bacterial panel that uses magnetic resonance to detect organisms within three to five hours. Two other features of the T2 panel make it valuable, Dr. Patterson says. “One is that it is more sensitive than a blood culture. And we’ve had cases where we couldn’t grow anything enough to identify it but the T2 did identify it. The other is that the T2 is not affected by antibiotics.”
Setting out with an MDW of 20 as a critical value at first created confusion among clinicians. “Things other than bacterial infection can cause the MDW to be high. If you keep telling them that this is critical and it doesn’t turn into anything, they’re going to stop paying attention to it.” To prevent this, the laboratory focused on education.
The hospitals used the MDW only in the emergency department to start because that’s how it was FDA cleared, Dr. Patterson says. Some ED patients would be admitted. “We wanted the doctors who were going to be handling them, once they left the ED, to know why some of the decisions were made,” so hospitalists and intensivists were educated too. For other clinicians, ”even though the MDW is being run on everybody, it’s being masked by the IT system” if it wasn’t ordered.
Clinicians have found the MDW useful because it starts falling when a treatment is effective, Dr. Patterson says. That was seen in a European study, which showed that, with patients who were identified as septic, “if their MDW started to decrease with treatment, those were the survivors.” The nonsurvivors were those in whom the MDW remained the same (Hausfater P, et al. Crit Care. 2021;25[1]:227).
The MDW is available only on Beckman’s DxH 900 and 690T hematology analyzers, says Zivjena Vucetic, MD, PhD, chief medical officer and senior vice president of medical and scientific affairs for Beckman Coulter. “It’s a CBC parameter and it measures distribution or dispersion of the volume of monocytes population in whole blood.”
Since MDW is separate from the CBC, “there was an analytical and clinical validation before the FDA clearance. There are numerous publications validating MDW’s use with patients who presented to the ED and had a CBC ordered. Together,” Dr. Vucetic says, “results of MDW and WBC tests outperform each test’s performance alone in terms of indicating the risk of sepsis in the next 12 hours.”
There is a small fee associated with the use of the MDW, says Jeff Tarmy, the company’s director of global publications and media. “But the fee is nominal when you consider all the benefits on the other side. And Beckman is the only company that offers this parameter,” he says.
There is more than one biomarker that is elevated in bacterial sepsis, Dr. Patterson notes, but the MDW parameter offers advantages. Since a CBC order is almost automatic for patients arriving in the emergency department and MDW is automatically performed as part of the CBC, there is no need to proactively order it. “Our thinking was that it could be particularly useful in the ED setting, because if the clinical criteria for sepsis weren’t met and if they’re not already thinking about sepsis and they saw the MDW was high, it would make them think about sepsis” and consider it a possibility in the differential diagnosis.
He and his colleagues don’t want clinicians to think an elevated MDW and an abnormal WBC automatically mean sepsis. But the patient is at a substantial risk for developing sepsis. “Normally, you draw blood cultures and you have to incubate them and that takes a while; it can be two or three days but at least overnight or 24 hours. Then, when your instrument alerts you that it’s positive, you take some of that infected blood and do a Gram stain and work it up and do more agar plates or a phenotypic platform to determine what the bacterium is.”
The T2 Biosystems panel instead uses magnetic resonance to detect the organism in the blood without culturing. “In our hands, it takes about 4.2 hours to get that result,” Dr. Patterson says. The weakness of the T2 at this point is that it detects only five organisms: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Escherichia coli. That covers about 70 percent of the sepsis cases. But “since we’re drawing the blood culture at the same time, you’re not going to miss the other organisms. You just won’t get them as fast.”
The infectious disease physicians suggested that the laboratory put a T2 kit together with a hold tube up front, but not run the MDW unless needed. “You draw it at the same time you draw the blood culture but from a separate draw—not out of a line but out of a vein. We have a reflex setup for when the MDW is high [above 20]. That, plus an abnormal WBC, alerts the microbiology lab to run the T2,” Dr. Patterson says. “Otherwise we just don’t use it; it gets discarded. That way, we don’t run a lot of unnecessary T2s.” Using this algorithm, he adds, allows for faster results to physicians, a better subset of patients selected for testing, and more appropriate antibiotic use.
He reports that a study of MDW stratification by diagnosis at three large EDs in the U.S. (Crouser ED, et al. Crit Care Med. 2019;47[8]:1018–1025) has been replicated in a Johns Hopkins Hospital study (Malinovska A, et al. J Am Coll Emerg Physicians Open. 2022;3[2]:e12679) and in Europe in the previously cited study of two large EDs in France and Spain (Hausfater P, et al. Crit Care. 2021;25[1]:227). All studies found that, based on sepsis-2 or sepsis-3 criteria, an MDW value of 20 is effective for sepsis detection, during the initial ED encounter, he says.
Dr. Patterson shared a few cases to illustrate Butler’s early experience in shortening time to diagnosis and appropriate therapy. In one, a 67-year-old male admitted with hypotension and altered mental status had no sepsis indicators but had an MDW of 23.98 and WBC of 24.7. A T2 test found P. aeruginosa, confirmed two days later by blood culture. The WBC plus MDW values saved 37 hours to targeted therapy.
In another case, a 70-year-old male with shortness of breath was admitted with hypothermia and hypertension and no sepsis indicators. He had a WBC of 23.2 and MDW of 23.05 and the T2 detected E. faecium, confirmed by blood culture 20 hours later, thus shortening time to targeted therapy by 20 hours.
In a third case, a 57-year-old female with altered mental status arrived at the ED with hypotension but had a WBC of 21.9 and MDW of 21.55 with a T2-detected S. aureus infection. In her case, the ED was able to get to targeted treatment 14 hours earlier than normal.
The shortened time to a species-specific diagnosis in these cases is actually even greater, Dr. Patterson says, “as these are times to a positive Gram stain on a culture rather than the complete species identification.”
“We have thousands of patients now on whom we’ve done this and this is a preliminary look at a few,” he says. The potential positive outcomes from use of MDW, in addition to an earlier, more appropriate treatment course, include an improvement in care through a shorter length of stay and fewer “bounce back” patients.
Based on the experience so far, Dr. Patterson reports that the hospitalists want to bring on MDW hospitalwide. That is the plan now. “We are about to go ahead and open MDW whole-house.” The laboratory has opted to stay with 20 as the cutoff value. “We thought if we were getting too many positives and not enough yield, we could raise it to 21. But that hasn’t turned out to be necessary.”
Ahead lies a potential expansion of the diagnostic algorithm. One possibility is use of technology from MeMed, maker of host immune response diagnostics. In a recent presentation to Dr. Patterson’s laboratory, “the company reported going through thousands of inflammatory markers like procalcitonin to come up with a group of markers that might help physicians decide if somebody does have a bacterial infection. And they have identified three of them, including tissue necrosis factor-related apoptosis-inducing ligand, interferon-gamma release assay, and C-reactive protein. I’d like to validate it and see how it works in our hands, but it’s potentially interesting.”
Also interesting is discovering what the cause of sepsis is, Beckman CMO Dr. Vucetic says. “We have collaborated and partnered with other companies that are differentiating between bacterial and viral infection as they develop what’s important in diagnosis.”
The standard tests used to assess sepsis risk are valuable, Dr. Patterson says. “When your white cell count goes up, your other inflammatory markers go up. It seems that when there is an infection, it causes activation of the monocytes that are already circulating in the body. So it doesn’t require a bone marrow response.” Sometimes lactate and procalcitonin are normal when they’re checked, but the MDW may not be. “We’ve even had cases where the white count was normal and everything else was normal except the MDW was high.”
Research has shown a gap, he says, between the sensitivity of MDW and that of inflammatory markers like lactate. The 2019 study by Crouser, et al. (Crit Care Med. 2019;47[8]:1018–1025), found a sensitivity of 0.75 for WBC, 0.79 for MDW, and 0.85 for MDW and WBC combined, while lactate sensitivity was 0.59. “Choosing the MDW gives you the largest yield in terms of detection,” Dr. Patterson says of the findings.
As to cost, the T2 is reimbursable, “but it does cost a bit more to run than you get reimbursed, for some unknown reason,” he says. “That’s another reason to try to limit it. You don’t want to do it on everyone.”
Beckman Coulter’s MDW parameter is used more widely in Europe than in the United States. It takes time to build awareness of a new parameter in laboratories and clinics, says Beckman’s Jeff Tarmy. And laboratories need a Beckman instrument to use MDW. Even for those with the instrument, “there is a significant time commitment that goes into researching, validating, and rolling out new tests. This can be a meaningful hurdle for labs that are already challenged with staffing shortages and tightening financial resources.”
“But MDW is a great tool that can overcome this legacy of poor adoption because it doesn’t need a discrete physician order to be reported and Beckman Coulter is equipped and experienced to support the clinical implementation of MDW,” Tarmy says. He says more than 300 sites in North America are using MDW.
The company continues to drive awareness and adoption of the parameter, Tarmy says. He agrees with the business analysts who project a significant increase in the sepsis diagnostics market over the next 10 years. Beckman will continue to expand its menu of tests, he says, and believes MDW has a big role to play in diagnosing and managing sepsis. The company’s objective is to expand the CBC’s value. “We believe there’s more clinical data available in the CBC, the most prescribed diagnostic we have. That is where we look at novel biomarkers,” he says.
Anne Paxton is a writer and attorney in Seattle.