Anne Paxton
September 2018—When a miracle drug comes along that is predicted to cause havoc in the laboratory, the drug could well seem like a double-edged sword. In the case of emicizumab (Genentech’s Hemlibra), for patients with hemophilia A, the mix of both benefits and drawbacks is likely to settle in for the long term.
Laboratories adjusting to the widening rollout of emicizumab will need to keep an important consideration in mind when performing coagulation testing for hemophilia patients, says Dorothy M. Adcock, MD, chief medical officer of LabCorp Diagnostics. “Emicizumab can interfere in APTT-based assays. The traditional factor VIII activity assay, the APTT-based one-stage assay, is not an accurate measure of patients’ level of hemostasis when they are on emicizumab.”

Dr. Levy
Emicizumab is a monoclonal antibody that represents one of the most dramatic advances in hemophilia treatment in decades. Approved by the FDA in November 2017, emicizumab could significantly reduce the treatment and disease burden of thousands of hemophilia A patients who have antibodies in their blood, known as inhibitors, that render standard factor VIII replacement therapies ineffective. Very soon, coagulation experts believe, the benefits of emicizumab will extend to all hemophilia patients.
“For patients with inhibitors, emicizumab is almost revolutionary in that they really haven’t had effective therapies to prevent bleeding,” says Guy A. Young, MD, director of the Hemostasis and Thrombosis Center at Children’s Hospital Los Angeles. He notes that patients with inhibitors have a 70 percent higher mortality rate than hemophilia patients without inhibitors. “In addition, those patients had a tremendous treatment burden—IV infusions three or four times a week or every day, with treatments lasting 45 minutes to an hour.” As a result, many did not adhere to the treatment. With emicizumab, patients with inhibitors will have less burdensome therapies that still lower their bleeding rates to those of patients without inhibitors who are on prophylactics, Dr. Young says.
“Bringing this medicine to patients is one of the things I am most proud of in my career,” Gallia Levy, MD, PhD, global clinical development lead for Hemlibra at Genentech, wrote in an email to CAP TODAY.
However, the benefits of emicizumab may come at the cost of laboratories’ ability to produce accurate coagulation test results for hemophilia A patients because emicizumab behaves differently from factor VIII in a key way: “Even the slightest concentration of emicizumab in the blood will normalize the activated partial thromboplastin time,” Dr. Young points out.
This means that activated partial thromboplastin time or any APTT-based test cannot be used in patients taking the drug, he says. “We will not be able to measure factor VIII levels accurately with the assays most available in the U.S. For the inhibitor patients, we will also not be able to track their inhibitor levels with the traditional assay. We may have a patient on emicizumab that is working fantastically to prevent their bleeding. That part’s great. But how we are going to be able to measure factor VIII activity and inhibitor titers is problematic currently.”
“That’s how emicizumab is going to create havoc in the laboratory, because lab tests that we normally would be comfortable doing and interpreting in hemophilia suddenly are no longer going to be accurate.”
While Genentech’s current U.S. product label outlines how emicizumab interacts with different coagulation tests and offers guidance on which tests are unaffected by the drug, coagulation testing experts interviewed by CAP TODAY point out there remains a critical awareness gap about the pitfalls of testing emicizumab patients.
Emicizumab reduces the bleed rate in hemophilia A patients with inhibitors via a simplified prophylaxis regimen that is patient-friendly: self-administered subcutaneous infusions once a week. In clinical trials of emicizumab, the drug produced a stunning difference in these patients’ bleeding levels. For instance, 54 of 57 patients (94.7 percent) under age 12 who received weekly subcutaneous emicizumab doses for bleeding prophylaxis had zero treated bleeds, in contrast with the typical pattern for these patients of monthly bleeds.
Personal stories have underscored the effect of emicizumab on hemophilia patients’ quality of life. “I know of one eight-year-old boy with factor VIII inhibitors who is very proud that he can administer his Hemlibra himself,” Dr. Levy says. Before Hemlibra, the boy needed infusions that could take up to two hours at least three times a week. The half-life of infused factor VIII is only 10 to 12 hours. Another pediatric patient cited by Roche, a co-developer of emicizumab, had experienced scores of debilitating ankle bleeds, but those bleeds stopped once he began receiving emicizumab during clinical trials.
Emicizumab was made possible by scientists at Chugai Pharmaceutical who had an idea, completely novel in 2000, of using a bispecific antibody to replace the function of factor VIII and help restore the blood clotting process, Dr. Levy says. “The scientists were already working on advances in antibody engineering and saw an opportunity to apply this approach to hemophilia A.”
The research leading to emicizumab entailed an extended, needle-in-a-haystack search. “Finding an antibody with the right configuration and properties to mimic factor VIII’s biological activity required screening more than 40,000 antibodies and took nearly 10 years. The molecule that would become Hemlibra was selected as the candidate for clinical trials in 2010,” Dr. Levy says. But all that effort led to a landmark in drug development: “Hemlibra is the first antibody medicine ever developed that replaces the function of a missing or altered protein.”
The clinical development of the drug was done as a partnership between Genentech, Chugai, and Roche (all members of the Roche Group). At the clinical trial phase, Genentech and Roche first studied how people with hemophilia A managed bleeds with their previous treatment options by gathering data in a noninterventional study. “Some of the people in this study went on to enroll in our pivotal studies of Hemlibra, allowing for a first-of-its-kind intrapatient analysis, the first time two prophylactic treatments have been compared prospectively in the same person in a hemophilia study,” Dr. Levy says. So the Hemlibra clinical trial program not only has had an impact on lives of people with hemophilia A but also may help influence the way hemophilia clinical trials are designed in the future, she points out. “In addition, a pivotal clinical trial, HAVEN 1, showed statistically significant results for 13 different bleed-related endpoints.”
Hemophilia A patients’ development of antibodies (inhibitors) to factor VIII replacement products is one of the most serious and common adverse events in hemophilia treatment, says Dr. Adcock. “Development of inhibitors occurs in about 33 percent of patients with severe factor VIII deficiency who receive replacement therapy. Once patients develop antibodies, it can be very difficult to treat them and very expensive. The cost of treating patients with factor VIII inhibitors is upwards of $1.5 million per year or more.”

Dr. Adcock
Patients receive factor VIII infusions as a prophylactic measure to prevent spontaneous bleeding or bleeding associated with trauma or everyday activities. But this therapy requires time-consuming intravenous treatment and, despite therapy, patients often experience bleeding into the joints, which can cause disabling arthritis. “The whole social impact is much broader than the cost of the drug,” Dr. Adcock says.
Until emicizumab’s entrance on the scene, clinicians had few effective recourses for patients who develop antibodies to factor VIII, Dr. Adcock notes. “Clinical protocols to treat these patients and to reverse the inhibitor response are not always effective, and they are very costly. One approach to eradicate the inhibitor is immune tolerance induction, where high doses of the replacement factor are administered on a frequent basis. The mechanism by which this may be effective in inhibitor eradication is not known.”
With emicizumab, the cost of treating patients with inhibitors could decline. In April, the nonprofit Institute for Clinical and Economic Review (ICER) released a final evidence report on emicizumab, which evaluated how emicizumab could improve health while lowering costs for certain people with hemophilia. The report concludes, “In assessing the value of treatments for hemophilia, payers should be aware of important benefits and contextual considerations that are not typically captured in cost-effectiveness analyses.”
“So even though emicizumab is expensive—about $430,000 per year—it still may be cost-effective in certain populations,” Dr. Adcock says. The report makes the case that third-party payers should cover emicizumab because, over time, it will reduce treatment costs. But the ICER report also warns that some controls on the price may be needed: “Given that emicizumab may gain indications for broader use, indication-specific pricing will likely be essential to tailor the price to reflect the clinical and economic value of the drug in different patient populations.”
Emicizumab works by replacing or mimicking the function of the missing cofactor, activated factor VIII, says Stefan Tiefenbacher, PhD, vice president of LabCorp and technical director of Colorado Coagulation, a member of the LabCorp Specialty Testing Group. “Emicizumab bridges factor IXa and factor X, bringing them into proper alignment—which is normally the function of activated factor VIII. However, there is a big difference between factor VIII and this bispecific antibody: Unlike factor VIII, which is highly regulated, emicizumab, once administered, does not require activation and circulates in the patient in its active form until it is cleared from circulation.”

Dr. Tiefenbacher
At the 2018 Scientific and Standardization Committee meeting of the International Society on Thrombosis and Haemostasis, Dr. Tiefenbacher, who develops drug-specific assays for clinical trials, presented a special session on practical considerations, including laboratory issues, that emicizumab raises.
This bispecific antibody has a long half-life of 28 days, he explains. “With more conventional hemophilia factor VIII replacement treatments, the ‘wash-out’ period of the factor product is usually a few days, maybe a week for the newer extended half-life products. Thus, you can safely perform a factor VIII inhibitor assay after a few days of wash-out without having to be concerned about the factor VIII replacement product interfering in the assay.” If, on the other hand, a hemophilia A patient is treated with emicizumab, the bispecific antibody circulates in the patient’s blood for up to six months after the last treatment, creating significant challenges in the laboratory when APTT-based factor activity and inhibitor are ordered, he says.
In APTT-based assays, emicizumab will result in a shortening, or normalization, of the APTT response, which in the one-stage factor VIII activity assay will lead to an artificial overestimation of the factor VIII activity level, as high as 500 percent or more. Emicizumab, even if present at very low concentrations, can thus give the appearance that a particular patient has either normal or even elevated circulating levels of factor VIII, Dr. Tiefenbacher says.
If laboratory professionals aren’t being educated and aren’t aware of these APTT-based assay interferences, he adds, they are at risk to report incorrect laboratory results, which could adversely affect patient care. For example, if the inhibitor level in a patient treated with emicizumab is monitored and reported using a standard APTT-based inhibitor assay, the assay will provide a false-negative result, which the treating physician could incorrectly interpret as the patient no longer having a measurable FVIII inhibitor titer, he says.
Genentech wanted to forestall this kind of false-negative by making an alternative assay available: the chromogenic two-stage inhibitor assay. “As far as chromogenic assays go,” he says, “there are currently five different reagents available in the U.S. Only two or three of these assays utilize bovine-based factor IX and factor X and have been demonstrated to be suitable for the measurement of factor VIII inhibitors in the presence of emicizumab. Should a laboratory use a chromogenic assay that utilizes human-based reagents, it also will be interfered with by emicizumab. Due to the specific design of the humanized bispecific antibody, it does not cross-react with bovine origin reagents, as the antibody was selected to be specific to human factor IX and X.”
When LabCorp receives hemophilia A patient samples for testing, the typical test order received is for a factor VIII activity and a factor VIII inhibitor. Dr. Tiefenbacher provides an example of how test results can go awry: “We had a patient who previously demonstrated with significantly elevated APTTs, factor VIII one-stage activities at around one percent or below, and with an increasing factor VIII inhibitor titer as high as 200 BU. Suddenly, a month later, this same patient presents with a normal APTT result—in fact, slightly below normal—and a corresponding factor VIII one-stage activity result of greater than 500 percent.
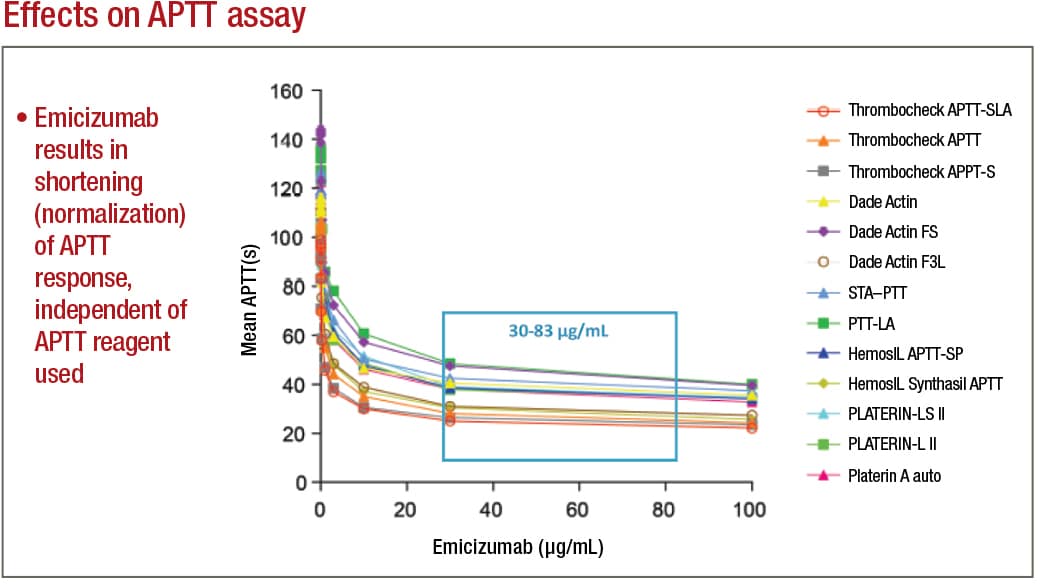
Adamkewicz, et al. Poster presentation at Hemostasis & Thrombosis Research Society, Scottsdale, Ariz., 2017; Courtesy of Genentech, Chugai, and LabCorp
“When the medical technologists brought these results to our attention, we suspected that the patient was switched from one of the traditional factor VIII replacement therapies to emicizumab. However, the treating physician appeared to have ordered the standard coagulation assays that are customary for patients receiving traditional factor VIII replacement therapy,” Dr. Tiefenbacher says. This same patient, when tested using a bovine reagent-based chromogenic assay, had nonmeasurable factor VIII activity. “We have since received several other patient samples from a variety of different hemophilia centers where the treating physician continued to order the standard lab testing associated with traditional factor VIII replacement therapy. Thus, there clearly is a need for additional education, both at the level of the ordering physician as well as at the laboratory level where the testing is performed,” he says.
Another potential future challenge for laboratories is that emicizumab is marketed as not requiring laboratory monitoring, he says. “However, one can come up with scenarios where laboratory measurement of emicizumab may be required, for example, when a patient requires surgery in an emergency-type setting. The treating clinician or surgeon and the laboratory will have to know that the patient is or has within the past six months been on emicizumab and that conventional APTT-based screening assays will provide inaccurate results.” It is Dr. Tiefenbacher’s understanding that some institutions are providing patients with bracelets that indicate the patient is on emicizumab therapy and that APTT-based assays cannot be used for monitoring, as is also stated on the drug label.
Since Colorado Coagulation is a reference laboratory, “we don’t always know what drugs the patient is taking,” Dr. Adcock says, “and the technologists in the lab have to exercise good judgment. When we see factor VIII activity that is extraordinary—for example, over 400 percent, where normal is up to 150 percent—then we question if the patient is on emicizumab, and we may reach out to the doctor or perform a chromogenic factor VIII activity assay.” As she explains, “If you want to obtain a semi-accurate activity of the emicizumab, you use the chromogenic human assay. To monitor factor VIII antibodies in patients on emicizumab, you use the bovine chromogenic factor VIII Bethesda inhibitor assay.”
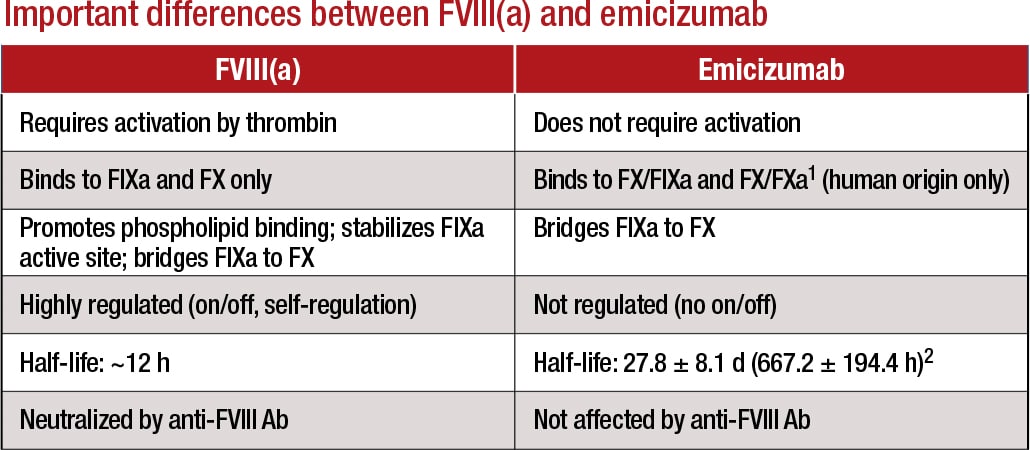
1Lenting, et al. Blood. 2017;130(23):2463–2468; 2Hemlibra FDA Prescribing Information, 11/2017 Courtesy of LabCorp
Dr. Tiefenbacher agrees: “We are a special coagulation lab, so we probably are more experienced in those areas, but it can take some detective work to determine if the observed interference in the patient sample on hand is due to emicizumab or some other type of drug or interference. Oftentimes we have little information provided for a particular sample other than the test order. This is where education and training of the involved parties becomes important.”
It’s also important to note, he says, that “until some type of neutralizer for emicizumab is developed, assay interference—at least in the APTT-based coagulation assays—should be anticipated for up to six months posttreatment. People need to be aware of this and use a bovine-based chromogenic assay when trying to measure inhibitors in these patients.”
Dr. Tiefenbacher makes five recommendations for coagulation laboratories addressing the testing needs of emicizumab patients: 1) avoid APTT-based coagulation assays such as APTT-based one-stage factor activity and factor VIII Bethesda inhibitor assays; 2) where possible, replace APTT-based assays with chromogenic or immunoassay-based coagulation tests; 3) use the chromogenic (bovine factor IXa and factor X) factor VIII Bethesda assay when measuring factor VIII inhibitors; 4) to measure emicizumab, use a chromogenic (human factor IXa and factor X) or dilute one-stage assay with emicizumab calibrator and controls; and 5) work closely with physician customers to provide education and training on emicizumab interference and the selection of the most appropriate tests.
Education will be crucial to avoiding serious adverse outcomes when laboratories test hemophilia patients, says Dr. Young, of Children’s Hospital Los Angeles. “The company that’s marketing the drug is working on educating the community. They are doing webinars and podcasts, conducting symposia at national meetings, and putting out flyers. But one problem will be, if patients are on the drug and somebody just decides, ‘I need a factor VIII level,’ without being aware of laboratory interference, they will misread the results and may make incorrect clinical decisions based on a test that should not have been done in the first place.”
The hazard is more there than anywhere else, he notes. “But my ultimate nightmare scenario is when, let’s say, a patient who has hemophilia and is on this medication is traveling somewhere, has an auto accident and major trauma, and is perhaps unconscious. They get taken to a trauma center and the center says, ‘Oh my gosh, you have pelvic fractures. We need to do surgery right away.’ The surgeons measure APTT to determine if there is risk for bleeding, but this patient who actually has hemophilia will have normal APTT and will be okayed to have major surgery.”
That’s going to happen, he predicts. “The patient will have surgery, and while emicizumab will offer some level of coagulation protection from bleeding, if it’s a big surgery it’s probably not going to be enough, and there will be significant bleeding during the surgery.”
If the drug becomes as widely used as he expects, “labs need to ask themselves when they are receiving samples if they should alter their requisition form,” Dr. Young says, so that when factor VIII activity level or inhibitor level is ordered, there is a box to check if the patient is on emicizumab. “If the lab is aware whether the patient is on the drug or not, that can help them in ensuring a proper test is done and proper interpretation is done.”
A double check, where a laboratory receives a sample and there is an alert asking if the patient is on emicizumab, is another possible mechanism that might help. “I think that’s something the laboratory societies should be considering,” he says.
The vast majority of hemophilia patients are treated at one of the 140 hemophilia centers in the U.S., Dr. Young says. “But there are some patients outside those centers, and I worry about those patients even more, because doctors may see emicizumab as really useful, and the sales rep will recommend that they put patients on it, which is fine if it’s beneficial to their patients. But the doctors may not be hemophilia or coagulation experts, and that’s where we need to worry even more about misinterpretation of lab results.”
Still, he says, it should not be forgotten that emicizumab is a life-changing drug. One example he cites is the case of a 12 year old who was spending 30 percent of his time in a wheelchair, missing a lot of school, spending a lot of time in the hospital. “Now, since he was put on the drug two-and-a-half years ago, he hasn’t missed any school, he pretty much has no bleeding, he can do all kinds of physical activities, and the family got rid of the wheelchair a long time ago.”

Dr. Young
Dr. Young has also seen the same effect on younger patients. “There have been four and five year olds who were bleeding all the time, having a lot of pain, and their families have to do a lot of infusions. Now I have five kids younger than age 12 who have been on the clinical trial of emicizumab an average of two years and none of them has had a bleed at all. They’ve gone from 15 to 20 bleeds a year, pain, damage to joints, and multiple IV infusions to basically being a normal kid and not having to do any infusions other than weekly subcutaneous ones.”
Soon, non-inhibitor hemophilia patients may also benefit from emicizumab, says Genentech’s Dr. Levy, pointing to exciting clinical trial results for Hemlibra in people without factor VIII inhibitors. “Our HAVEN 3 study showed that Hemlibra prophylaxis resulted in a statistically significant and clinically meaningful reduction in treated bleeds compared to no prophylaxis. It also showed Hemlibra is the first medicine to significantly reduce bleeds compared to prior factor VIII prophylaxis, the current standard-of-care treatment for people without factor VIII inhibitors, as demonstrated by a statistically significant reduction in treated bleeds in an intrapatient comparison” (Mahlangu J, et al. N Engl J Med. 2018;379[9]:811–822).
Based on the data from the HAVEN 3 trial, Genentech and Roche filed a supplemental biologics license application and were granted priority review for Hemlibra to treat adults and children with hemophilia A without factor VIII inhibitors. The FDA is expected to announce a decision on approval of this widened application in early October, Dr. Young says.
In the meantime, Genentech continues to address the issue of accurate test results by stressing education. “Education of patients and physicians is something that we take very seriously and will continue to do,” Dr. Levy says. “Separately, we are working to support the continued development of existing tests that may be of use to the hemophilia community, as well as new tests that may be of help to physicians in certain clinical scenarios.”
Anne Paxton is a writer and attorney in Seattle.