Karen Lusky
December 2017—Having validation data to support the use of age-adjusted D-dimer cutoffs with the D-dimer assay your laboratory uses is a must, and know well the limitations of point-of-care prothrombin time/INR testing.
That advice and more was shared in a “Hot Topics in Hemostasis” session at CAP17, presented by Russell Higgins, MD, and Karen Moser, MD.
The D-dimer test has attracted attention of late because age-adjusted D-dimer cutoffs are part of an American College of Physicians clinical guideline for ruling out acute pulmonary embolism.
The ACP guideline authors took an “algorithmic approach to all steps of pulmonary embolism diagnosis, starting with assigning a clinical prediction score,” said Dr. Moser, an assistant professor of pathology at Saint Louis University School of Medicine and a member of the CAP Coagulation Resource Committee (Raja AS, et al. Ann Intern Med. 2015;163[9]:701–711). In general, they recommend patients with a low or intermediate pretest probability of pulmonary embolism undergo D-dimer testing. Those who have a high pretest probability should proceed to imaging.
“Contained within this overall document,” Dr. Moser said, “the ACP guideline tells us that clinicians should use age-adjusted D-dimer thresholds, defined as the patient’s age times 10 ng/mL, rather than a generic cutoff of 500 ng/mL, in patients older than 50 years to determine whether imaging is warranted.”
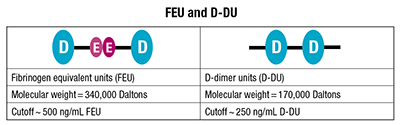 The ACP guideline didn’t stipulate the type of unit for the proposed age-adjusted D-dimer (AADD) cutoff, which could be fibrinogen equivalent units (FEU) or D-dimer units (D-DU). Two FEUs are equal to one D-dimer unit. In published comments, the ACP clinical guideline authors subsequently said their intent was FEUs. (See “FEU and D-DU.”)
The ACP guideline didn’t stipulate the type of unit for the proposed age-adjusted D-dimer (AADD) cutoff, which could be fibrinogen equivalent units (FEU) or D-dimer units (D-DU). Two FEUs are equal to one D-dimer unit. In published comments, the ACP clinical guideline authors subsequently said their intent was FEUs. (See “FEU and D-DU.”)
Where did the age-adjusted D-dimer cutoff come from? The most often cited study, Dr. Moser said, is called ADJUST-PE, “a cute acronym” that stands for looking at AADD cutoffs in patients over 50 for the diagnosis of pulmonary embolism. The study was a prospective, multicenter validation of AADD cutoffs in patients in that age group (Righini M, et al. JAMA. 2014;311[11]:1117–1124).
The ADJUST-PE researchers didn’t just “pull this cutoff out of thin air,” Dr. Moser said. “They had previously done retrospective work looking at patients in their centers’ populations and subjected those historic data to ROC curve analysis. And it’s just really fortunate for them that what appeared to be the best fit for an age-adjusted cutoff also happened to be really easy to remember: age × 10 ng/mL.”

Dr. Moser
As is standard practice in this patient population, the researchers measured D-dimer in patients who had low to intermediate clinical pretest probability established by Wells or Geneva scoring, Dr. Moser said. “Different centers used different clinical prediction scores. And they used six different D-dimer assays that are commonly used in clinical practice in the laboratory with these cutoffs of either 500 ng/mL or 0.5 µg/mL FEU.”
The study findings were promising. The failure rate, defined as the number of patients who on three-month follow-up were found to have a thromboembolism on imaging after they had a negative AADD result, was 0.3 percent. “So that failure rate—or the three-month risk of VTE in patients who were less than the AADD cutoff—was essentially equivalent to the rate of VTE in patients who had D-dimers less than the manufacturer’s cutoff of 500 ng/mL, or negative pulmonary angiography,” Dr. Moser said. “So they performed comparably, which is encouraging.”
In addition, the AADD cutoff yielded a fivefold increase in negative D-dimer results in patients older than 75. “These are patients who often have renal failure and other comorbidities,” she noted. “They might not be great candidates for some of the contrast-assisted imaging we use.” She cautioned that the study had a limited number of patients of that age.
“The ADJUST-PE results seem to bear out in other trials,” Dr. Moser added, citing a smaller study conducted within one emergency department using five similar D-dimer assays (Mullier F, et al. Blood Coagul Fibrinolysis. 2014;25[4]:309–315). A meta-analysis of 13 studies, published before ADJUST-PE was published, also supported the use of AADD cutoffs (Schouten HJ, et al. BMJ. 2013;346:f2492).
Members of the CAP Coagulation Resource Committee shared their thoughts on the ACP guideline in an article published earlier this year (Goodwin AJ, et al. Ann Intern Med. 2017;166[5]:361–363). “Basically I would say the committee’s response is to point out that D-dimer reporting has been confusing for a long time, and it’s critically important to specify the unit type when you are discussing D-dimer values so that anyone who is looking at the values knows exactly what you are talking about,” Dr. Moser, a coauthor of the article, tells CAP TODAY.
The other challenges the CAP committee saw from the laboratory perspective, Dr. Moser says, is that any age-adjusted D-dimer cutoff a laboratory selects has to have validation data to support use of that cutoff with the specific D-dimer assay the laboratory uses. “So whether those data come from large-scale clinical studies in the literature using the laboratory test kit, or whether that comes from an internal laboratory study, there has to be something to support use of the cutoff,” she says. “You cannot just take the proposed cutoff values from the ACP guideline and say, ‘This is going to work with my assay.’ You need to have data to support any cutoff you use in your laboratory.”
In their article, the CAP committee members proposed a short-term strategy recommending that clinical laboratories interested in using AADD cutoffs “consider only specific D-dimer assays adequately evaluated in clinical studies based on the CLSI guidelines,” referring to CLSI approved guideline H59-A, “Quantitative D-Dimer for the Exclusion of Venous Thromboembolic Disease,” published in 2011. The committee also recommended a long-term strategy of harmonizing D-dimer assays and reporting by improving assay performance and unifying reporting units.
The ACP clinical guideline authors said in response, in published comments, that they were referring to “the most commonly used and robustly studied Fibrinogen equivalent units in the ACP Best Practice statement.” They also said, “If D-Dimer Units are used, multiplying the result by ten for patients above 50 years old underestimates the value; rather, providers should consider multiplying by twenty and considering the result in the context of the normal range of their testing laboratories.” They agreed with the Coagulation Resource Committee’s recommended short- and long-term strategies. These and the remainder of their comments were published March 21, 2017 on the Annals of Internal Medicine website.
Dr. Higgins, former chair of and now advisor to the Coagulation Resource Committee, says multiplying the result from assays calibrated in D-DU by 20 does not make sense and does not correlate to the published AADD cutoff calculation. “This is terribly confusing,” he said in an interview, “and it exemplifies the real-world concerns the CAP Coagulation Resource Committee has with the universal application of AADD cutoffs across all assays.” Calculations performed in the laboratory must be handled with caution, he adds, and the calculations by the treating physicians “are equally problematic.”

Dr. Adcock
Dorothy M. Adcock, MD, chief medical officer of LabCorp Diagnostics, calls the AADD cutoffs a “good idea.” Baseline D-dimer levels increase with age. “So the older we get after about 40 to 50 years of age, the higher our D-dimer rises. And this is probably due to underlying atherosclerotic vascular disease. It’s a very well-documented phenomenon,” says Dr. Adcock, an author of the CLSI H59-A guideline on D-dimer (John Olson, MD, PhD, was chair) and lead author and chair of the CLSI H54-A guideline on INR calibration.
Dr. Adcock predicts that use of AADD cutoffs will take off. “I think the article that the CAP Coagulation Resource Committee published in Annals of Internal Medicine will help promote that because they identified studies that were specific for different manufacturers’ kits.” Laboratories using those kits named in the article will therefore be more inclined to report the age-adjusted values.
In the CAP17 session, a poll of the audience was taken. “I would say a minority of participants indicated this was something their clinical colleagues are asking them about,” Dr. Moser says. “But certainly now that this idea has made it into a clinical practice guideline for one of the major internal medicine organizations, I think this is something that laboratories are going to be increasingly facing as the advice is disseminated and the news gets around to other specialties—family medicine and emergency medicine as well as internal medicine.”
The University of Utah hospitals and clinics are using and have been reporting the age-adjusted D-dimer cutoffs since mid-2016, says Chris Lehman, MD, medical director of the clinical laboratories and clinical professor of pathology, University of Utah School of Medicine. The laboratory implemented the AADD cutoffs, Dr. Lehman says, because it found out from the medical director of the internal medicine thrombosis service that clinicians were already interpreting numeric results on their own without verification from the laboratory that the test was one that was included in recently published clinical trials and meta-analyses, “and without verification that they were uniformly applying the correct multiplication factor.”
The laboratory reports the D-dimer results as µg per mL fibrinogen equivalent units. The lab information system was set up to compute the cutoff based on the patient’s age by year. “The numerical result is reported with the calculated cutoff with a note stating that a D-dimer result less than the cutoff is considered a negative result,” Dr. Lehman says.
Before making the change, Dr. Lehman discussed the published literature with the thrombosis service medical director to confirm they both agreed there was sufficient data to justify supporting use of the age-specific cutoffs, and that the lab’s D-dimer assay was adequately represented in the published studies.

Dr. Lehman
The CAP Laboratory Accreditation Program 2017 checklist requirement (HEM.37925) says if a different cutoff than what is provided in the manufacturer’s package insert will be used, adequate data must be cited to support it, Dr. Lehman says. “For cut-off data acquired from the literature,” it says, “a negative predictive value of ≥98% (lower limit of CI ≥95%) and a sensitivity of ≥97% (lower limit of CI ≥90%)” are recommended for non-high pretest probability of venous thromboembolism. These recommendations come from the CLSI H59-A guideline, he says.
How large a study would a laboratory have to do on its own? In the CLSI H59-A document, Dr. Moser says, “the recommended study design for validating a VTE exclusion cutoff includes three months follow-up of the included patient population, which should constitute at least 200 patients, and you need to have correlation with imaging studies at the time of diagnosis and over that three-month follow-up period.”
At the University of Utah hospitals, Dr. Lehman hasn’t heard of any concerns or problems with the use of the AADD cutoffs. The medical director of the thrombosis service did report that the positive predictive value of CT scans ordered specifically to rule out pulmonary embolism appears to have increased since the implementation of the age-adjusted cutoffs. The implementation, Dr. Lehman notes, coincided with an institutionwide initiative to increase adherence to recommended clinical guidelines for ruling out PE.
Dr. Lehman’s advice to other laboratories rolling out AADD cutoffs: “Make sure your LIS or EMR can support the calculations required, work with your clinician experts, and use the CAP hematology and coagulation checklist for guidance.”
Oksana Volod, MD, director of the coagulation consultative service and an associate professor of pathology at Cedars-Sinai Medical Center, Los Angeles, recalls that she had just seen the ACP clinical guideline article in 2015 when the emergency department chair sent her the same article by email and said the department believed that type of strategy would decrease unnecessary imaging and the rate of false-positives. From there, “It was escalated to the leadership of the pathology department that [the AADD cutoffs] had to be brought on board,” says Dr. Volod, also a member of the CAP Coagulation Resource Committee.
First the laboratory changed its D-dimer ranges, cutoffs, and units according to the manufacturer preferred units, she says. Second, they examined whether they were comfortable with the age-adjusted D-dimer cutoffs. The answer proved to be yes. They use the STA-Liatest D-Di (Stago) quantitative immunoturbidimetric assay, and in an international multicenter study (D-Dimer for the Exclusion of Thromboembolism, or DiET), the assay was shown to surpass the CLSI/FDA guidance requirements for a D-dimer assay, Dr. Volod says. “It was also used in clinical studies of AADD cutoffs for pulmonary embolism exclusion.”

Dr. Volod
“We switched in late 2015. We report in the new way and we do have age-adjusted cutoffs. However, we didn’t allow the clinicians to calculate the number,” which the lab information system does automatically. “Physicians and physician leadership were notified about the changes via various educational venues.”
The impact on patient care has been similar to what Dr. Lehman reports. The chair of the emergency department told Dr. Volod no follow-up has been conducted, “but he can clearly see there’s a significant reduction in unnecessary imaging, and they do not have patients, for example, who are discharged and readmitted with a pulmonary embolism. So I think it did improve the specificity [of the D-dimer testing].”
Dr. Volod expects the impetus for implementing the AADD cutoffs to come from the emergency departments in large institutions. “Maybe smaller institutions will not be hit by this,” she says, “but I do believe that major centers that have a trauma center and an emergency department will, because it is the major topic during big conferences and it is best-practice advice from the clinical guidelines committee of the ACP. And we have to be ready for it.”
The laboratory has to know whether it can do it, Dr. Volod says. “And if they can do it, they need to communicate how it will be done and how it will be reported.”
Point-of-care PT/INR testing has been in the hot seat for a number of reasons, among them a highly publicized recall of an INR monitoring system and other FDA-related issues. (See “Are the point-of-care PT/INR devices safe and effective?” page 38.)
Dr. Higgins encouraged the CAP17 audience to think of the laboratory INR as different from a point-of-care INR, which he noted has unique features. “First of all, we use whole blood in point of care often through a fingerstick. As the blood is traveling through that wound, it is exposed to tissue factor and the clotting process is beginning. So you really have to get that drop of blood on the meter as quickly as possible so you get the right answer. That’s something we don’t deal with in the laboratory,” said Dr. Higgins, associate clinical professor, Department of Pathology and Laboratory Medicine, University of Texas Health Science Center at San Antonio, and medical director of UHS Pathology Services.
Dr. Higgins presented a vignette involving a 45-year-old male patient who was bridged from low-molecular-weight heparin to warfarin. The patient had a point-of-care INR result of 5.2. The central lab INR was 2.7.
Low-molecular-weight heparin was the likely culprit. Dr. Higgins said the POC devices may not contain heparin neutralizing substances: “Central lab INRs do contain substances that neutralize heparin, usually up to one unit per mL, and they can be used for bridging warfarin from heparin therapy. But the point-of-care devices shouldn’t be used in this way.” He also stressed that point-of-care or laboratory INRs cannot be used to monitor any of the direct oral anticoagulants.
Lupus anticoagulants can affect both the central laboratory method and the POC method, which varies again by the device used, Dr. Higgins cautions. “The way we deal with this in our warfarin clinic is anybody who is being anticoagulated for antiphospholipid syndrome would just get a venous draw that’s sent to the central lab,” he said, noting that the POC devices’ responsiveness to lupus anticoagulants isn’t well studied, so the central laboratory has more experience with them.
 Whole blood INRs and POC INRs are influenced by hematocrit, but a study Dr. Higgins cited showed hematocrit had only a small effect on POC INRs. The patients studied had a mean INR of 3.13, and their hematocrits ranged from 37 to 51 (van den Besselaar AM, et al. Thromb Haemost. 2008;100[6]:1181–1184). The authors wrote, “The magnitude of the effect of haematocrit, within the reference interval of 0.37–0.51, on the INR difference was not greater than approximately 10% for the combined data of the four strip lots.” A bias of less than 10 percent appears to be clinically acceptable, they added.
Whole blood INRs and POC INRs are influenced by hematocrit, but a study Dr. Higgins cited showed hematocrit had only a small effect on POC INRs. The patients studied had a mean INR of 3.13, and their hematocrits ranged from 37 to 51 (van den Besselaar AM, et al. Thromb Haemost. 2008;100[6]:1181–1184). The authors wrote, “The magnitude of the effect of haematocrit, within the reference interval of 0.37–0.51, on the INR difference was not greater than approximately 10% for the combined data of the four strip lots.” A bias of less than 10 percent appears to be clinically acceptable, they added.
Dr. Higgins reported that Wayne Chandler, MD, and colleagues “took it a step further” and demonstrated that when you get up to INRs of about 5, the whole blood INRs actually are related to the hematocrit (Amukele TK, et al. Am J Clin Pathol. 2010;133[4]:550–556). “While WBINR [whole blood INR] testing may be sufficiently accurate below an INR of 4,” Dr. Chandler and colleagues wrote, “higher WBINR values need to be repeated by a plasma method.”
“Depending on exactly how the INR instrument for point of care is set up, it can be sensitive to what the hematocrit is,” Dr. Chandler, division chief of laboratory medicine, Department of Laboratories, Seattle Children’s Hospital, tells CAP TODAY. So one would need to know the sensitivity of the particular device to hematocrit, and most people don’t, he says. “The endpoint of this is that it is another potential mechanism of interference and why a point-of-care device could potentially give you a different INR than a standard lab test based on plasma only.”
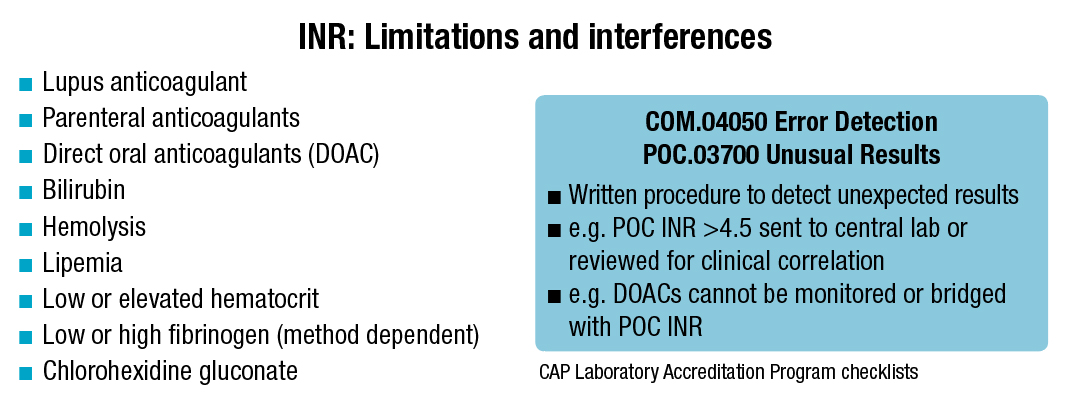
UT San Antonio uses point-of-care PT/INR testing solely for monitoring warfarin in the clinic, Dr. Higgins says. If the clinic gets an unusual result—usually an INR above 4.5—it correlates it to a central laboratory result. “The clinical correlation that can be done in the warfarin clinic can be important, too, to verify a high INR,” he says. (See “INR: Limitations and interferences.”)
 In his talk, Dr. Higgins reiterated that POC PT/INR is FDA approved for monitoring warfarin. “Most of the package inserts that I have looked at,” he says, “have very narrow language stating that the indication for the test is for monitoring warfarin. Only a very few of the point-of-care PT/INR [devices] have more open language in the package insert that would potentially allow them to be used for acutely ill patients.”
In his talk, Dr. Higgins reiterated that POC PT/INR is FDA approved for monitoring warfarin. “Most of the package inserts that I have looked at,” he says, “have very narrow language stating that the indication for the test is for monitoring warfarin. Only a very few of the point-of-care PT/INR [devices] have more open language in the package insert that would potentially allow them to be used for acutely ill patients.”
The clinical indications for which point-of-care PT/INR testing has been studied include, among many others, acute ischemic stroke, liver transplantation, acute hemorrhage, and acute traumatic coagulopathy. One study was performed in remote locations of Australia, Dr. Higgins said, where it would be helpful to have a POC PT/INR device to detect snakebite coagulopathy. Yet in the study three of the seven snakebite victims with coagulopathy would have been missed by the POC device, which had an electrochemical endpoint detection method. “The lab INR is elevated,” he said. “The point-of-care INR is normal” (O’Rourke KM, et al. Thromb Res. 2013;132[5]:610–613). The INR testing was performed using iStat POC devices.
“I love the snake study,” Dr. Higgins says, “because it’s a great example of where the point-of-care PT/INR was very different from central lab PT/INR when the intended use was changed to detection of venom-induced coagulopathy. So it drove home the point that a point-of-care PT/INR is not equivalent to a central laboratory PT/INR.”
Explaining why that was so in the snake study, Dr. Higgins showed an illustration representing the extrinsic pathway of the coagulation cascade, which he noted culminates in the generation of thrombin. “Normally what we see is thrombin acting on fibrinogen, which then causes a fibrin clot to be formed. And then we detect that either mechanically or optically in the laboratory. In the point-of-care instruments that use this electrochemical endpoint, thrombin actually acts on a reagent. So when the reagent is cleaved by thrombin, it releases an electroactive chemical and that’s detected amperometrically. (See “Electrochemical endpoint and fibrinogen.”)
“So it really has nothing to do with fibrinogen at all,” Dr. Higgins said. The method has been shown to be insensitive to low fibrinogen in the Australian snake study and in another study, he added. The latter used Roche Diagnostics’ CoaguChek XS (Kim SJ, et al. J Clin Lab Anal. 2015;29[1]:28–31). The researchers said their “results indicate that the use of CoaguChek XS INR without meticulous matching to laboratory INR may cause problems in certain conditions associated with hypofibrinogenemia.” The conditions include blood loss, hemodilution, sepsis, disseminated intravascular coagulation, and chronic liver diseases.

Dr. Higgins
The authors of the study acknowledged they used citrate plasma “both for the CoaguChek XS and laboratory INRs despite the fact that the CoaguChek XS is designed to use capillary blood from a finger prick. However, it has been repeatedly reported,” they wrote, “that plasma has been used successfully for external quality assessment purposes to detect discrepancies between these devices.” They also noted that not all the study patients were on oral anticoagulation therapy.
Corinne R. Fantz, PhD, director of medical and scientific affairs for point-of-care business at Roche Diagnostics, says Roche isn’t in accord with some of the authors’ conclusions as stated in the study. For one, the researchers “used a highly artificial sample material [recalcified citrate plasma] instead of whole, nonanticoagulated blood,” she says. “It’s possible that the recalcified plasma caused an interference with the electrochemical measurements, so it’s difficult to assess whether or not similar results would be seen with the intended use sample type.”
Roche monitors customer feedback and reporting regarding its devices, and since the CoaguChek XS system was introduced in 2006, she says, low fibrinogen has not been a common complaint. “None of the performance data we have with the intended use sample indicate the system is affected by low fibrinogen.”
Some of the study’s conclusions could be attributable to the clinical scenario, Dr. Fantz says, in particular whether patients are coming from an outpatient or inpatient environment. “Clinicians in the outpatient environment would likely be aware of a congenital hypofibrinogenemia diagnosis before enrolling patients in a warfarin management program, or they may discover it during the initiation period,” Dr. Fantz says. It’s more common in an inpatient environment to see acquired causes of fibrinogen, such as liver disease, hemodilution, or massive hemorrhage. “In those cases, it’s unlikely the meter is being used to monitor warfarin patients,” she points out. “If the meter is being used in an off-label manner, the institution would have to validate its use as a high-complexity test to avoid potential issues.”

Dr. Chandler
The ICU and emergency department are the settings in which patients are acutely ill and might have low or elevated fibrinogen or a low or high hematocrit that can interfere with the POC PT/INR device results, Dr. Higgins says. “What I worry about is that those rare patients who have a low fibrinogen due to DIC would be getting a different point-of-care PT/INR result . . . and that’s why I think it’s important for pathologists to understand the differences in these tests.”
In Dr. Chandler’s view, if you have a clinic and are seeing a stable patient who comes in weekly with the same fibrinogen and the same hematocrit and everything else, “then these point-of-care devices can be quite useful and they can get you a result quickly.” But if something is changing in the patient—the person is sick or something else has happened—“then that’s when you need to just step back and say, ‘Wow, there are a lot of things going on in this patient, and I’m not sure if I trust this value. It might be good to check it with the lab as well.’”
Dr. Higgins presented a case from many years earlier involving a complaint from the warfarin clinic. The clinic said its POC device and the lab INRs were not in agreement. Point-of-care INRs of 5 or 5.4 were about 7 in the laboratory.
The values did appear to be different, Dr. Higgins conceded, but he displayed a bias plot that shows that when INR values climb above 4.5, there becomes a large bias (difference) between methods. “When we have INRs that are above the therapeutic range [for warfarin], we start to see biases, and that’s just to be expected,” he said.
“This is not just the point-of-care instruments. This is also true for central lab INRs,” he added. “This is kind of a limitation of the INR, so it’s not a limitation of the devices or the instruments we have. It’s not really ethical to collect patients who are all supratherapeutic on warfarin to develop a certified plasma that has an INR of 9.”
Medical directors can implement numerous quality activities to limit the risks at every stage of POC PT/INR testing, Dr. Higgins noted. “In the preanalytic phase, we make the standard operating procedures reflect the limitations of the assay, stating, ‘We don’t monitor warfarin in antiphospholipid patients. We don’t monitor direct oral anticoagulants.’
“In the postanalytic phase, if we make it very clear that when we get high INRs we are going to send another sample to the lab for verification, then we are sort of limiting risk.” He reminded attendees that in the analytic phase of the POC PT/INR testing, they have to follow the manufacturer’s instructions for performing quality control. He favors also performing proficiency testing for waived PT/INR devices: “You know it’s another way to see if our devices are performing as expected or if something is wrong.”
Karen Lusky is a writer in Brentwood, Tenn.
[hr]
Is it acceptable to convert units?
Clinical laboratories are allowed to convert fibrinogen equivalent units (FEU) to D-dimer units (D-DU) and vice versa, but should they?
Karen Moser, MD, of Saint Louis University School of Medicine, says the CAP checklist requirement “strongly suggests” laboratories report patients’ D-dimer results using the same unit (FEU or D-DU) recommended by the manufacturer in the product insert. “If the lab decides to report in different units, they need to verify conversions annually and with any change in reagent or instrument,” says Dr. Moser. “The conversion must be verified for patient results, cutoff values, and reference intervals, and a record of verification should be maintained.”
Why might labs choose to convert units? Dr. Moser notes that laboratories coming together in a network may not be using the same D-dimer kit when they merge. “And from a laboratory perspective, we always want to provide the most seamless experience for our clinicians,” she says. “You could imagine in a situation like that the laboratories saying, ‘Well, it’s easier for us to make a conversion so that no matter which hospital site our clinical colleagues are working in, they’re seeing what to them looks like a uniform D-dimer result with the same units, and they are using the same cutoffs.’”
“There is a significant safety benefit,” Dr. Moser agrees, “in terms of preventing confusion, to providing D-dimer results in the same units with the same VTE [venous thromboembolism] cutoff to a group of physicians practicing in a given network. But I think the best way to do that is to standardize the D-dimer kit in use across laboratories in the network, given the many pitfalls possible in D-dimer unit conversion.”
Dorothy M. Adcock, MD, of LabCorp Diagnostics, says some labs convert units because they historically used manufacturer A, which reported in D-dimer units, and then they transitioned to manufacturer B, which reports in FEUs. To avoid confusing their clinicians, the labs convert their FEUs to D-dimer units. “But this is really not recommended,” she says, noting that the units as stated in the package insert are the units that should be used for reporting.
—Karen Lusky
[hr]
Are the point-of-care PT/INR devices safe and effective?
December 2017—Safety issues related to point-of-care PT/INR testing surfaced in recent years, among them a 2016 voluntary class 1 recall of Alere’s INRatio and INRatio2 monitor systems. “Prior to that, the company that manufactured the device had received thousands of complaints about it,” says Russell Higgins, MD, of the University of Texas Health Science Center at San Antonio.
“What is known is that some patients who may have been kind of ill would get lower INRs, and so theoretically they could be given more warfarin than they should have. They’d be supratherapeutic even though their INR was maybe in the therapeutic range. That was the risk. And it was thought to be related to when the patient was ill and perhaps related to elevated fibrinogen that can [accompany] acute illness. But as far as I know,” Dr. Higgins says, “nobody ever did a study to really show that fibrinogen was the problem.”
A medical device correction issued by Alere on Dec. 5, 2014 cited several contraindications to using the INRatio PT/INR monitor system, including anemia and “any conditions associated with elevated fibrinogen levels.”
The INRatio device was used in the ROCKET AF (atrial fibrillation) trial, Dr. Higgins says, and the trial generated some of the data submitted to the FDA for the clearance of the direct oral anticoagulant rivaroxaban, which can be used in lieu of warfarin (Cohen D. BMJ. 2016;352:i575). Many thought it was possible that the patients in the warfarin arm seemed to have more bleeding because they were receiving more warfarin than they should have based on an INR measured with the INRatio, Dr. Higgins said. “And that made the rivaroxaban appear more safe.” The FDA, manufacturers, and other organizations went back and analyzed the clinical trial data and concluded that rivaroxaban is safe.
Another safety concern: “The FDA had been under fire for some time regarding its 510(k) process for approving medical devices,” Dr. Higgins says (Meier B. NY Times. July 29, 2011). “If you have a new device and you show that it is substantially equivalent to a predicate device, then you can get approval without doing sort of these complicated clinical studies to get it approved.”
These events led to the FDA putting on a public workshop on POC PT/INR devices in March 2016. Dr. Higgins, who was the chair of the CAP Coagulation Resource Committee at the time, presented a session titled, “POC PT/INR: Technical Limitations and Laboratory Accreditation Issues.” The goal of the meeting, according to the FDA, was to identify solutions to address the scientific and regulatory challenges associated with point-of-care PT/INR devices to ensure safety and effectiveness.
Says Corinne Fantz, PhD, of Roche Diagnostics: “We heard from lab experts, clinician experts, FDA experts, etc. Everyone has a unique lens they use to look at this problem the FDA is trying to address, which is to ensure that point-of-care PT/INR instruments are safe and effective.”
“When doing studies as a manufacturer, we want to ensure we have all the right information for the FDA to complete their assessment,” Dr. Fantz says. “Once the FDA suggests a standard, that’s what we work toward, whether it’s in draft or final form.”
Dr. Fantz says the FDA did suggest specific accuracy standards that were “different than the criteria by which current instruments are cleared.
“For example, previous criteria were 90 percent of samples in the INR range from 2.0 to 4.5 needed to match the reference method within 30 percent, and there were no criteria above an INR of 4.5. The new proposal is 95 percent of samples within 20 percent of the reference method and 25 percent above an INR of 4.5.”
In the future, Dr. Higgins predicts, the FDA may be “tightening its criteria for evaluating the substantial equivalence of a new device against an existing device.” In his view, the 510(k) process can be problematic for point-of-care PT/INR devices in that it is assumed that all devices that measure PT/INR are similar. But at least five different types of endpoint determinations are used in the POC devices (Higgins RA, Hemostasis. In: Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 5th ed.). “To me, that makes the devices for the INRs different tests,” he says. “Those tests are susceptible to different interferences and limitations because they have different mechanics, different endpoint determinations.”
FDA spokesperson Deborah Kotz informed CAP TODAY in an email that the agency “is continuing to work on guidance on POC PT/INR devices. We are also working on efforts to better educate consumers about how to properly use these devices to monitor warfarin. We cannot comment on the timing of our future actions but this remains a big priority for us,” she wrote.