Karen Lusky
December 2016—Barry Nelson was the first in his cancer patient support group to undergo immunotherapy, which at the time was in a phase one clinical trial at Dana-Farber Cancer Institute. As the therapy became available to others in his group, they would ask for his advice on whether to try it. For the answer, he’d suggest they consult with their doctors and pray. He’d add, “I hope if you decide to go with it that you’ll have the same results that I do.”
The PD-1 inhibitor that Nelson received in the trial from 2014 to February 2016 shrunk his advanced non-small cell lung cancer by more than 25 percent. Nelson told attendees at the CAP16 plenary session in September that the cancer is still present but not growing. “And I have my life. I can continue to have a full life and do everything I wanted to do,” he said.
For Nelson, the PD-1 inhibitor, pembrolizumab, was fifth-line therapy. He’d had standard chemotherapies and radiation and participated in an EGFR clinical trial, although Nelson’s cancer didn’t have the EGFR mutation, said Christopher S. Lathan, MD, MS, MPH, who is Nelson’s clinical oncologist at Dana-Farber.
Dr. Lathan, Nelson, and pathologist Lynette M. Sholl, MD, were panelists in the plenary session on the pros and cons of immune checkpoint inhibitors and their future prospects.
Dr. Sholl, panel moderator and associate pathologist and associate director of the Center for Advanced Molecular Diagnostics at Brigham and Women’s Hospital, said, “We’ve been surrounded by really incredible stories like Barry’s of extraordinary outcomes in terminal cancer patients who miraculously are given a second chance at life.”
“On the flip side, these success stories are dampened by concerns about the drug costs, which are quite steep, and speculation about profiteering, and the sobering realization that the cancers treated with these therapies, even in patients who have a great response, may go on to evolve resistance.”

Dr. Sholl
Dr. Sholl, who is also an assistant professor at Harvard Medical School, noted that PD-1 curtails bodily damage created by the immune-mediated response to an infection or inflammation. A variety of immune cells express this receptor. “And when programmed death ligand one, or PD-L1, binds to PD-1 expressed on the T cells, it blocks these immune responses. So although cancer cells can express antigens that should attract the attention of the T cells in the neighborhood, by overexpressing PD-L1, cancer cells essentially hijack the normal immune checkpoint mechanisms and evade this attack,” she explained. “Antibodies targeting these interactions release these brakes and permit the T cells to do their job.”
“The clinical benefit of this approach to cancer killing became clear initially when trials using antagonists against CTLA-4 showed a remarkable benefit in patients with metastatic melanoma,” she added.
Within the past 12 to 18 months, immunotherapeutics have received FDA approval or breakthrough status in head and neck squamous cell carcinoma, melanoma, non-small cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, urothelial cancer, and mismatch repair deficient colon cancer.
Response rates, according to data Dr. Sholl shared, range from more than 80 percent for Hodgkin lymphoma to 15 percent for bladder carcinoma. Melanoma has a 30 to 40 percent response rate and MMR deficient colon cancer a 40 to 70 percent response. For NSCLC it is about 20 percent, for renal cell carcinoma about 25 percent, and for head and neck carcinoma, 15 to 25 percent.
How long do patients respond? Gordon J. Freeman, PhD, of Dana-Farber Cancer Institute and a professor of medicine at Harvard Medical School, tells CAP TODAY that a study conducted by F. Stephen Hodi, MD, and Jedd D. Wolchok, MD, PhD, of how melanoma patients who used the CTLA-4 drug were faring years later found that about 20 percent of them were living and had a good response, which was evident by the second year. Patients don’t take the drug after the first year, he says. “If the patient was alive at year three, they were also still alive at year 10,” says Dr. Freeman, whom Dr. Sholl described as instrumental in discovering PD-L1’s role in cancer and developing “a class of therapeutics.” (Dr. Freeman was not a plenary speaker.)

Dr. Freeman
“The PD-1 drug hasn’t been in use as long as the CTLA-4 drug,” Dr. Freeman says, “so we don’t yet know if the results will be as long-lived, but the early results are promising.” Dr. Hodi and Dr. Wolchok reported that 35 percent of melanoma patients treated with a PD-1 drug were alive at five years. “So that’s better than the CTLA-4 drug.”
“We don’t know whether we can extrapolate what happens in melanoma to lung cancer,” says Dr. Lathan, an assistant professor of medicine at Harvard Medical School. “Melanoma is more immunogenic than lung cancer, just as breast cancer is less immunogenic than lung cancer.” Some patients whose lung cancer recurred after they stopped taking a PD-1 inhibitor have responded to a re-treatment with the drug, he adds.
PD-L1 expression on tumor cells has been identified as a biomarker to predict response to the PD-1 and PD-L1 drugs, Dr. Sholl said. “The knowledge of PD-L1 expression in the tumor cells is required for use with certain drugs and not for others.”
There appears to be “a kind of metered response curve,” Dr. Lathan says, “meaning that as a trend, those who have a higher expression of the biomarker tend to be people who have better and more durable responses.” Yet there are patients with low PD-L1 expression who respond to the drugs and others with high expression who do not.
Dr. Freeman says some patients with no PD-L1 expression by assay have “a low but real chance to respond” to a PD-1 inhibitor. How might that occur? A second PD-1 ligand, PD-L2, which he and research colleagues have demonstrated, could be involved, he says. “Also, the assay isn’t perfect. When you do the PD-L1 assay, you stick a needle into one little bit of a tumor, and it’s been shown that one area of a tumor can be different from another area. Another possible reason,” Dr. Freeman says, “is that there is no PD-L1 in the tumor but there is PD-L1 elsewhere in the immune system and the assay doesn’t look at the PD-L1 elsewhere. The PD-L1 could be on the immune cells in the lymph node, which is not tested in a needle biopsy.”
In the plenary, Dr. Sholl said she and Dr. Lathan thought oncologists probably were not depending that heavily on a biomarker because nivolumab can be used as second-line therapy without an accompanying biomarker test. “But in the first line, if pembrolizumab becomes approved in that context, we will need to know the PD-L1 status,” she said. (On Oct. 24, the FDA gave pembrolizumab the go-ahead for front-line use for NSCLC. See page 50, “Recent approvals and the pipeline.”)
Dr. Lathan discussed some of the challenges to using the PD-1 inhibitors for lung cancer patients. “We are in this very odd space with our tissue [where] we spent years and years trying to get smaller biopsies to make it better for patients. And now we’re asking you guys, ‘By the way I’ve got to get PD-L1, EGFR. I want ALK. I want you to do all the IHC so I know exactly what I’m dealing with.’ And then we’re going to send it out again for a trial sometimes requiring another biopsy. And I think that that’s a lot. It’s asking a lot of patients.”

Dr. Lathan
An audience member asked him if there’s any role to advocate for obtaining a bigger specimen up front for many of the tests eventually requested. “The short answer to that is yes,” Dr. Lathan said. “We do try to get core biopsies instead of FNAs.” But it depends on the practice setting and the patient’s health. If someone is 65 to 75 years old with COPD, and the interventional radiologist has concerns about injuring the patient, it’s easier to get an FNA than a core in those scenarios, he said.
“Personally, I always advocate whenever I can,” he continued. “When I’m up front, I try to talk to the interventionalist and let them know if it’s possible, get more cores.”
Dr. Lathan has also found that the way in which PD-L1 results are reported can be confounding. One of his patients was interested in entering the nivolumab and ipilimumab clinical trial. The patient had PD-L1 testing performed at an outside hospital, which reported it was “40 percent negative but it was negative.” “We don’t know what that means,” he said. “And it doesn’t mean anything for ipilimumab. And so we’re just stuck with this information that’s very hard to interpret.”
Then there’s patient misperception of the treatment. In Dr. Lathan’s view, the best thing about the drugs is their side-effect profile. But one thing that has been “disconcerting,” he said, is when patients request the drugs because they see them in the lay press and tend to view immunotherapy as natural and chemotherapy as a poison. “They say, ‘You’re turning our own body on the cancer. It just feels right, doesn’t it?’”
The truth, he said, is that the PD-1 inhibitor side effects can be toxic, and only about 20 percent of patients are going to respond to the drug. “I do think sometimes in the rush to push folks over to immune therapy, especially in the first-line setting, we might be missing some of the effects of cytotoxic chemotherapy. For good or for ill, sometimes cytotoxic chemotherapy can be very effective.”
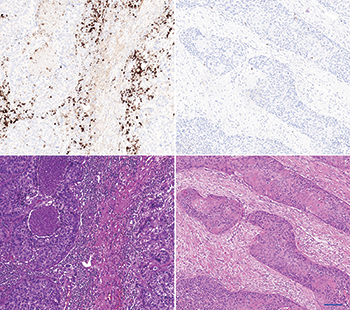
The left panel shows an “inflamed” carcinoma. The upper left shows a strong PD-L1 immune cell staining by SP142/ Ventana. The lower left shows the corresponding HE stain. The stroma is cell rich and contains a high density of immune cells. The right panel shows an “immune desert” carcinoma. The upper picture shows a negative PD-L1 immune cell staining by SP142/ Ventana. The lower right shows the corresponding HE stain. The stroma is fibrous and contains fibroblasts without immune cells. Scalebar is 100 µm.
Dr. Sholl discussed other potential biomarkers for predicting which patients might be helped by the PD-1 and PD-L1 inhibitors. “Some groups have described the correlation between the tumor mutational burden and response to immune checkpoint blockade,” she said. “And others have proposed a combination of tumor and immune cell profiling to define a ‘hot signature’ that may predict response to these drugs. As we all know, the best biomarker must be firmly grounded in science but must also be accessible to pathologists in diverse practice settings.”
Dr. Sholl asked Dr. Lathan in the plenary how things such as tumor mutation burden, “which actually correlate with a patient’s smoking history and potentially with KRAS mutations,” affect his decision to consider immunotherapy for a particular patient in the second or later line. Eighty-five percent of lung cancer patients have had tobacco exposure, Dr. Lathan noted, and 25 percent have a KRAS mutation. “I just don’t operationalize that,” he said, adding that he stores such information as “potentially useful in the future.”
Tumors that don’t respond to a PD-1 inhibitor don’t seem to have many T cells when the pathologist looks at them, Dr. Freeman says. “The idea is that the PD-1 drug doesn’t start the immune response from nothing but frees a smoldering but inhibited immune response to be effective. It’s allowing the T cells already in the tumor to be more active and attack the tumor,” he explains. “An idea people are focusing on now is how do you get the tumor to be infiltrated with T cells that can attack it?”
In a separate Roche-sponsored talk at CAP16 on PD-1 inhibitors and the tumor microenvironment, Mark Kockx, MD, PhD, a pathologist and medical director at HistoGenex in Belgium and Naperville, Ill., showed images (at left) that underscore how important it is to see if a tumor has ongoing inflammation or if it is noninflamed, which is also called an immune desert. Many factors lead to this immune desert condition, he said in an interview with CAP TODAY. “Cell types and moieties within the stroma of the tumor microenvironment,” such as cancer-associated fibroblasts, can induce substantial immunosuppression.
Dr. Kockx postulates that the resistance biomarker for PD-L1 and PD-1 checkpoint drugs may be the presence of cancer-associated fibroblasts in the tumor, in addition to an immune desert and an absence of PD-L1 expression. “The pathologist is necessary to manage this nuanced complexity,” he says.
At the moment, looking for inflammation in and around the tumor is how groups of patients tested for PD-L1 expression are potentially “bucketed” in pathology, agrees Roger Dansey, MBBCh, senior vice president of global clinical development oncology, Merck Research Laboratories. “I think our focus is not so much on the histologic appearance,” he tells CAP TODAY, “but more at looking at changes in genes so we can group patients using RNA expression analysis into those that represent the inflamed responders, inflamed nonresponders, and the noninflamed nonresponders.” (He notes that the latter would be equivalent to the immune desert.) “One of the challenges in introducing new testing is that you want something that’s highly reproducible—and gene expression profiling could be an alternative option to IHC,” Dr. Dansey says.

Dr. Dansey
“Biological pathways,” says Dr. Kockx, “also contribute to immunosuppression—for instance, the angiogenic pathway where the integral VEGF molecule itself has an immunosuppressive action. These elucidations provide a rationale to combine anti-angiogenic drugs like Avastin with checkpoint receptor inhibitors, and therefore convert an immune desert to an immune active environment.”
Radiation or conventional chemotherapy might also do the trick. “Inducing cancer cell death,” Dr. Kockx says, “can increase immunogenicity by exposing tumor neoantigens to the immune system. This in turn triggers response from the immune cells, which we have observed in samples following chemotherapy or radiation—an increased influx of immune cells.”
There’s another potential tactic. Merck and partner Amgen are conducting clinical trials in which the researchers inject an oncolytic virus directly into tumors. Says Dr. Dansey: “The virus is genetically modified to express granulocyte-macrophage colony-stimulating factor, or GM-CSF. The combination of the virus that causes lysis of tumor cells plus the local release of GM-CSF which attracts macrophages and other cells into the tumor microenvironment—and the addition of Keytruda given systemically—is the same sort of concept as chemotherapy or radiation where you are causing injury in the tumor that’s allowing an inflammatory response to develop and then taking the brakes off the immune response with PD-L1 inhibition and looking for improved outcomes.”

Dr. Kockx
Dr. Kockx points to two other immunotherapy modalities: personalized vaccines and engineered T-cell therapy. The identification of cancer neoantigens, although still novel, will be important to identify candidates for either of these methods, in his view. (In T-cell therapy, T cells are removed from a person’s body, trained against the cancer antigen in the laboratory, and then reinfused into the person, he notes.)
The two modalities also offer combination strategies with PD-1 and PD-L1 inhibitors, which have been shown in melanoma, Dr. Kockx says. “This method still requires surveillance of the tumor microenvironment, since a tumor landscape containing immunosuppressive elements will mount formidable resistance against vaccines or engineered T cells.” By systematically interrogating the tumor microenvironment with a combination of morphology and molecular technologies, he says, “we can develop a treatment strategy to leverage the supportive effects of the tumor microenvironment while evading or eliminating the suppressive elements.”
“Now that PD-1 has opened the door,” Dana-Farber’s Dr. Freeman says, “people realize the immune system can successfully attack cancer. There is just an incredible amount of energy and enthusiasm going into scientific studies in academia and the pharmaceutical industry to find things that work with PD-1 to increase the success rate, to extend it to other tumor types in a combination to do better. It’s abundantly clear that there are combinations that work together to do even better.”
Recent approvals and the pipeline
Worldwide, Dr. Freeman says, there are 20 PD-1 or PD-L1 drugs in 803 clinical trials with 166,736 patient slots ongoing. “We will find out what works best, what’s safest, who it works for, how it works, and how to cure many cancers.”
[hr]
Karen Lusky is a writer in Brentwood, Tenn.