Anne Paxton
March 2016—It’s not every day that a monoclonal antibody leads the news. But when former president Jimmy Carter was successfully treated for metastasized melanoma last year with the new drug pembrolizumab (Keytruda), the story made headlines. Carter’s recovery—surprising to many when it was announced in December—may have been helped by traditional radiation and chemotherapy. However, the role played by pembrolizumab spotlighted immunotherapy as an exciting advance in the evolution of cancer treatment.
As its use widens, immunotherapy is ushering in a new leading role for pathology in cancer diagnosis and treatment, and pathologists will need to adapt, says Kenneth J. Bloom, MD, head of oncology and immunotherapy for genomics information technology company Human Longevity in San Diego.
Presenting in a CAP webinar in December, Dr. Bloom detailed how immunotherapy can interrupt the phenomenon of immune checkpoint blockade and the kinds of challenges and dilemmas this process is posing for pathologists. “Instead of focusing only on the tumor and trying to delve deeper and deeper into a small sample from it, now we’re beginning to recognize that the microenvironment might be as essential—and maybe even more essential—for strategies for killing the tumor,” Dr. Bloom says.
People must shift their thinking from targeted therapy—tailored to mutations and the therapies that might best suit them—to immunotherapy, he believes. Because tumor cells have such strong adaptability, they can escape from genomically targeted agents. Immunotherapy addresses this by using the body’s own defenses to allow T cells to expand and kill tumor cells in a variety of malignancies.
Pembrolizumab is one of four major immunotherapy compounds that have either been approved by the Food and Drug Administration or are in phase three trials, and all have a companion diagnostic partner, Dr. Bloom says. Atezolizumab (Genentech) and durvalumab (AstraZeneca) use antibodies from Ventana, while nivolumab (Bristol-Myers Squibb) and pembrolizumab (Merck) use antibodies from Dako. The first two are targeted against PD-L1 (programmed cell death protein 1 pathway) and the second two are targeted against PD-1.
Matching up each drug with a diagnostic involves many more parameters than is typical with targeted therapy, and that can create confusion for pathologists. Testing depends on the indication, Dr. Bloom says. “When a physician asks us to determine PD-L1 status to aid them in determining the efficacy of a particular drug, we need to know not just which drug but what tumor type and the intended use.”
“This is because the basis for the drug approvals might differ; it could be overall survival, progression-free survival, or overall response rate. We also need to know the type of tumor. Is this for a lung cancer and, if so, what type—squamous or nonsquamous? Is this for a melanoma? For bladder cancer?” Then, he adds, the pathologist needs to determine what assay to use, what cells to assess—possibly both tumor cells and immune cells—and what cutoff to use.
“That’s a lot of questions. So when a physician comes up and says, ‘I want to order a PD-L1 test,’ I need to know the answers to all of those questions to best inform them not only which test will be most predictive of response to therapy, but also so that I understand how, as a pathologist, I should interpret the results of that assay.”
With targeted therapy, the pattern has been that the FDA approves the molecule for one indication, generally in a metastatic second- or third-line setting. “Then after a couple of years we see an approval in a first-line setting,” Dr. Bloom says.

Dr. Bloom
With immune drugs, things are happening much faster. Pembrolizumab, for example, an anti-PD-1 drug, was given accelerated approval for unresectable or metastatic melanoma and disease progression following treatment with Yervoy and, if BRAF V600 mutation-positive, a BRAF inhibitor. It has approval, too, for patients with metastatic non-small cell lung cancer whose tumors express PD-L1 as determined by an FDA-approved PD-L1 assay and who have disease progression on or after platinum-containing chemotherapy, as well as for patients with EGFR or ALK genomic tumor aberrations and who have disease progression on FDA-approved therapy for these aberrations prior to receiving pembrolizumab.
Nivolumab has already been approved for unresectable or metastatic melanoma as a single agent (for both BRAF V600 wild-type and mutation-positive tumors) and in combination with Yervoy, as well as for patients who have metastatic NSCLC who have disease progression on or after platinum-containing chemotherapy. More recently it was approved for advanced renal cell carcinoma in patients who have received prior anti-angiogenic therapy.
“These immune drugs appear to be active in so many different tumor types that the rationale for using the drugs, and the approvals for these drugs, will extend over time,” Dr. Bloom says.
In the case of pembrolizumab, data showing an improvement in survival or disease-related symptoms were not initially presented to the FDA. But “that doesn’t mean there wasn’t a survival benefit,” Dr. Bloom says. “The response to the drug looked so beneficial that it got approved before there was data on survival benefit.” Those data were published recently.
When the ongoing trials are completed, the number of indications is likely to expand, but the cutoffs for those indications might change. “For each of the drugs, there is also a unique PD-L1 immunohistochemical assay also approved. Both are produced by Dako. They are pharmDX 28-8 for nivolumab and pharmDX 22C3 for pembrolizumab.”
The trial leading up to the pembrolizumab approval was a phase one study designed to determine the optimal dosage to give, Dr. Bloom says. “They were looking at overall response rate by RECIST criteria and also some secondary endpoints. What was demonstrated was an excellent response rate, either partial response or stable disease, in about 45 percent of patients overall.”
Patients who had a tumor proportion score of more than 50 percent—meaning more than 50 percent of the tumor cells were showing some level of expression of PD-L1—showed a significant response rate and duration of response compared with those who had either less expression or no expression of PD-L1, he notes. “It was on this basis that FDA approval was given.”
 The lack of serious side effects was impressive. “If you focus on the grade 3 to 5 adverse events, there were very few that occurred in more than one percent of the patients, and those adverse events—pyrexia, elevation in aspartate aminotransferase, and anemia—are easily treatable. These are incredibly low numbers. The most significant adverse event that you see is fever in about 3.8 percent of patients, but most of the bad side effects are in far less than one percent of the population.” This makes pembrolizumab an extremely well-tolerated drug, especially compared with chemotherapy, he adds.
The lack of serious side effects was impressive. “If you focus on the grade 3 to 5 adverse events, there were very few that occurred in more than one percent of the patients, and those adverse events—pyrexia, elevation in aspartate aminotransferase, and anemia—are easily treatable. These are incredibly low numbers. The most significant adverse event that you see is fever in about 3.8 percent of patients, but most of the bad side effects are in far less than one percent of the population.” This makes pembrolizumab an extremely well-tolerated drug, especially compared with chemotherapy, he adds.
The concept of tumor proportion score, or TPS, was important for the study of PD-L1 pharmDX 22C3 antibody, Dr. Bloom says. “Based on detailed analysis of the clinical trial data, three categories were implemented. ‘No PD-L1 expression’ meant that less than one percent of the tumor cells expressed PD-L1, low expression meant between one and 49 percent of the tumor cells expressed PD-L1, and with high PD-L1 expression, 50 percent or more of the cells showed staining.”
The TPS is the same as the percentage of tumor cells showing staining; that is, number of stained tumor cells divided by the total number of tumor cells. The calculation can get a little tricky because of the difficulty in estimating the number of tumor cells reproducibly and the heterogeneity of PD-L1 expression. (See Fig. 1.)
“If we’ve got a big piece of tumor, it is suggested to break that up into quadrants, calculate the total proportion score in each quadrant, then add them up and divide by four,” Dr. Bloom says. That process works most of the time, if there’s relatively equal distribution of tumor cells in each quadrant. “But if there are wild differences between the number of tumor cells in each quadrant, then you have a more complex calculation.”
Staining patterns can be difficult to read at times, he notes. In the upper left image of Fig. 2 “when it looks like a HER2 stain and we see a strong circumferential thick membranous stain, that’s not a problem. Even in the lower left, where we see basolateral staining, it is relatively easy. It’s where we see some cells with light, granular, or poorly localized staining that it’s difficult to tell.”
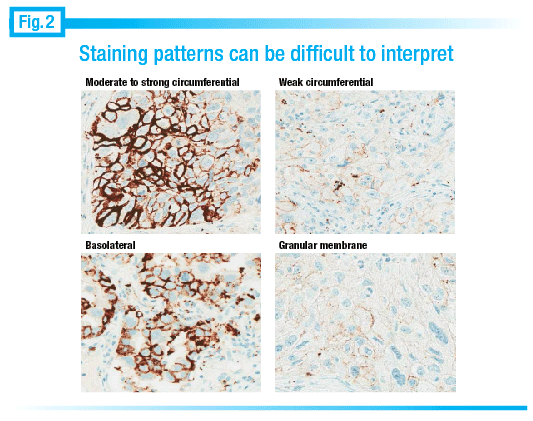 It can be challenging to figure out whether the staining is, in fact, real. “What we’re really looking for is stain that’s localized to the membrane. So cytoplasmic staining doesn’t count; extracellular staining doesn’t count.” In the lower right image of Fig. 2, he notes, “It’s a little bit tough to tell whether that granular staining is really in the cytoplasm or just sitting on the membrane.”
It can be challenging to figure out whether the staining is, in fact, real. “What we’re really looking for is stain that’s localized to the membrane. So cytoplasmic staining doesn’t count; extracellular staining doesn’t count.” In the lower right image of Fig. 2, he notes, “It’s a little bit tough to tell whether that granular staining is really in the cytoplasm or just sitting on the membrane.”
It’s also important to distinguish tumor cells from tumor-associated immune cells because PD-L1 can be expressed on both of them, Dr. Bloom says. “Differentiation can be very challenging in lung cancer where you can have a post-obstructive pneumonia, histiocytes in alveolar spaces lined by tumor, and lymphocytic infiltrates that can strongly express PD-L1.”
“We want to count any membrane staining, at any level of intensity, whether it’s partial or complete, not just the strong ones. Even if there’s very weak staining, as long as we can see it clearly on the membrane, we should call it positive.” Sometimes this can be what he likes to call “hallucinatory staining”—that is, “if you’re not sure whether it’s there or not. It should be clear and distinct membrane staining. Most of the time it’s nice and linear and easy to classify, but sometimes it can be very weak or granular and quite challenging to figure out what percentage is actually on the membrane, versus nonmembranous, which wouldn’t count as positive.”
When the staining is present in significantly more than 50 percent of the tumor cells, interpretation is fairly straightforward, Dr. Bloom says. But with lower TPS scores, interpretation can be problematic. “You probably want somebody with some experience in looking at PD-L1 immunostains to read these assays. Not every stain is perfect, so before you read the slide you definitely want to ensure there is <1+ background staining. High background staining would prevent you from appropriately interpreting the slide.” (See Fig. 3.)
As in all immunohistochemistry, edge artifacts exist and need to be avoided, Dr. Bloom notes. “Necrosis can be particularly problematic, as can crush artifacts. You may have some tumor cells next to the necrosis, but you want to be sure the necrosis itself is ignored since it frequently shows strong expression.”
Dr. Bloom is pleased that his laboratory has been making findings in line with the pembrolizumab clinical trials. “When we looked at the results from the pembrolizumab trial [KEYNOTE-001], the percentage of patients demonstrating ≥50 percent expression in their tumor cells was about 22 to 23 percent overall, and to date in my laboratory patients’ samples show expression in ≥50 percent tumor cells between 25 and 30 percent of the time.”
 “So it is nicely in line with where the clinical trial was. If we look at the tumor proportion score that would be called negative [< 1 percent], that represented about 40 percent of the lung cancer patients. Preliminary data on the duration of response, overall survival, and progression-free survival with pembrolizumab from the KEYNOTE-001 trial showed the greatest benefit in the ≥50 percent category, with the one to 49 percent and the < 1 percent showing about equal benefit overall.”
“So it is nicely in line with where the clinical trial was. If we look at the tumor proportion score that would be called negative [< 1 percent], that represented about 40 percent of the lung cancer patients. Preliminary data on the duration of response, overall survival, and progression-free survival with pembrolizumab from the KEYNOTE-001 trial showed the greatest benefit in the ≥50 percent category, with the one to 49 percent and the < 1 percent showing about equal benefit overall.”
Since the preliminary study, data are now available for the KEYNOTE-010 study (Herbst RS, et al. Lancet. Published online ahead of print Dec. 19, 2015), which included a comparator arm. “In the KEYNOTE-010 study, two doses of pembrolizumab were compared against the standard chemotherapy, docetaxel. The recent publication reported that overall survival was improved over docetaxel with PD-L1 expressions ≥1 percent, and progression-free survival was superior at expression ≥50 percent.”
These findings leave up in the air where the cutoffs should be set. “Given the improvement in overall survival, maybe the new cutoffs are going to be one percent, not 50 percent. We’ve got to wait for the clearances to come along from the FDA.”
Nivolumab (Opdivo) studies are also proving provocative. With nivolumab, the study designs took a different approach from the original pembrolizumab research and are intriguing to pathologists, Dr. Bloom says. “One of the early nivolumab studies, called CheckMate 017, was a second-line study in stage IIIb/IV squamous cell cancers. They compared nivolumab versus docetaxel, very much as the KEYNOTE-010 did with pembrolizumab, but the KEYNOTE-010 study included all non-small cell lung cancers.”
“When we look at the benefit [Fig. 4], especially the overall survival benefit compared to chemotherapy, it was quite significant. There was a one-year median overall survival with nivolumab of 9.2 months versus 6.0 months with chemotherapy.”
Interestingly, for every category of PD-L1 expression, there was a benefit of nivolumab over docetaxel. “So it didn’t make a difference whether your tumor was PD-L1 positive or PD-L1 negative.” In its trials, Bristol-Myers Squibb used three different cutoffs for positivity—≥1 percent, ≥5 percent, and ≥10 percent—in an attempt to better define what level of PD-L1 expression predicted response, if any. PD-L1 expression didn’t make a difference in predicting response to nivolumab in squamous cell NSCLC. There was a benefit of nivolumab over docetaxel, even in PD-L1 negative patients.
 That says there’s benefit, for squamous cell cancer, to receiving nivolumab over docetaxel independent of PD-L1 status. “And when we looked at the toxicity level, again, there were very few grade 3 and grade 4 toxicities or adverse events. One significant adverse event was pneumonitis. Side effect profiles are likely to become very important as oncologists consider therapy options.”
That says there’s benefit, for squamous cell cancer, to receiving nivolumab over docetaxel independent of PD-L1 status. “And when we looked at the toxicity level, again, there were very few grade 3 and grade 4 toxicities or adverse events. One significant adverse event was pneumonitis. Side effect profiles are likely to become very important as oncologists consider therapy options.”
Nivolumab clearly demonstrates benefit when compared with chemotherapy. “This was a big deal. Nivolumab was the first inhibitor really demonstrating survival benefit versus the standard of care, with a 41 percent reduction in death,” Dr. Bloom says.
Taking the same drug and applying it in nonsquamous cancers yields similar but not identical results.
Findings presented last year at the American Society of Clinical Oncology annual meeting demonstrated that nivolumab doubles survival for PD-L1 expressors. “When we look at the data based on PD-L1 expression in nonsquamous NSCLC, we find that PD-L1 expression becomes important.However, the patient gets a benefit from nivolumab at even a one percent level of PD-L1 expression, versus what would be called negative expression. It just doesn’t make a significant difference whether you put the cutoff at one percent, five percent, or 10 percent benefit, but there is some minor improvement in benefit as the cutoff is raised. Survival curves of patients showing no expression of PD-L1 are basically identical whether they received docetaxel or nivolumab.”
This means the two drugs have about equal efficacy for survival in PD-L1 negative tumors. “But remember, the safety profile for nivolumab had very few grade 3 and grade 4 toxicities compared to chemotherapy. So you have similar survival in PD-L1 negative patients but a much better safety profile.” In addition, for those patients who had a response with either nivolumab or docetaxel, the duration of response was significantly higher with nivolumab—17.2 months for nivolumab versus 5.6 months for docetaxel, he says. (See Fig. 5.)
In the traditional scenario, the FDA reviews biomarker data on drug targets along with data from the clinical trial to assess if a classic companion diagnostic assay needs to accompany the drug label. All patients who were on KEYNOTE-001 pembrolizumab were assessed for PD-L1 status with the 22C3 kit. Approval was based on the overall response rate of approximately 45 percent as well as the duration of the response in patients whose tumor showed PD-L1 expression in at least 50 percent of the tumor cells. “Therefore, the approval of pembrolizumab was based on being screened as positive with the 22C3 kit, and the kit was approved as a companion diagnostic for the drug.” The 50 percent cutoff level was based on overall response rate and the duration of the response, not overall survival, he adds. But he tentatively expects the cutoff for that indication will probably reduce to one percent.
 For nivolumab, the FDA introduced a different concept: complementary diagnostic. “They approved the pharmDX 28-8 kit with nivolumab for nonsquamous NSCLC but they labeled the test a complementary diagnostic, meaning testing provides additional information about how a drug might be used but testing is not essential for the safe and effective use of that drug. Tumors showing expression of PD-L1 with the pharmDX 28-8 kit were more likely to receive a significant benefit from nivolumab versus docetaxel, but even patients lacking PD-L1 expression had a benefit similar to docetaxel but with less side effects.”
For nivolumab, the FDA introduced a different concept: complementary diagnostic. “They approved the pharmDX 28-8 kit with nivolumab for nonsquamous NSCLC but they labeled the test a complementary diagnostic, meaning testing provides additional information about how a drug might be used but testing is not essential for the safe and effective use of that drug. Tumors showing expression of PD-L1 with the pharmDX 28-8 kit were more likely to receive a significant benefit from nivolumab versus docetaxel, but even patients lacking PD-L1 expression had a benefit similar to docetaxel but with less side effects.”
Use of the pharmDX 28-8 kit is not required for patients with nonsquamous NSCLC, but PD-L1 testing with the kit provides complementary information that allows the physician to make a better-informed decision about which drug to use. “So it can be a little confusing. If a patient presents with metastatic squamous cell lung cancer and the oncologist is considering the use of nivolumab, PD-L1 testing is unnecessary since there is a clear survival advantage versus chemotherapy regardless of PD-L1 status. But if the patient presents with metastatic nonsquamous lung cancer, PD-L1 testing may be informative.”
 Whether nivolumab or pembrolizumab is better alone or in combination with other drugs is another question on which the jury is still out. “Looking at first-line therapy in unresectable stage III/IV melanoma and comparing the progression-free survival of patients treated with nivolumab versus the combination of nivolumab plus Yervoy” [ipilimumab, approved by the FDA Oct. 28, 2015], “we see some interesting things.”
Whether nivolumab or pembrolizumab is better alone or in combination with other drugs is another question on which the jury is still out. “Looking at first-line therapy in unresectable stage III/IV melanoma and comparing the progression-free survival of patients treated with nivolumab versus the combination of nivolumab plus Yervoy” [ipilimumab, approved by the FDA Oct. 28, 2015], “we see some interesting things.”
“The combination therapy improves PFS over nivolumab alone and is significantly better than just Yervoy. When stratified by PD-L1 expression, patients with negative tumors do significantly better with combination therapy versus either one. But patients with PD-L1 positive [≥5 percent] tumors appear to do the same whether they receive nivolumab or the combination.”
The importance of that finding again has to do with the safety profiles. “The safety of nivolumab by itself is very favorable, but patients get significantly more grade 3 and grade 4 adverse events when combination therapy is used. It might be necessary,” he says, “to do PD-L testing in the future using a different cutoff or possibly not at all.”
Anti-PD-L1 drugs are likely to be approved soon and their benefits remain to be seen as clinical trial data become available, Dr. Bloom notes. The anti-PD-L1 drugs “may have some theoretical benefit over anti-PD-1 drugs, but we will have to wait for the results of the clinical trials to see if the benefits are real.” For example, atezolizumab was engineered to remove antibody-dependent cellular cytotoxicity function, which results from natural killer cells recognizing antibodies bound to the targeted molecule and destroying the cell expressing it. In a small series of NSCLC, no cases of serious pneumonitis were noted but “we’re still waiting for more data to emerge,” Dr. Bloom says. A more significant issue related to the anti-PD-L1 drugs is that they were studied using two different PD-L1 assays with different scoring systems, both manufactured by Ventana. The SP142 assay developed as a companion to atezolizumab includes evaluation of both the tumor component and the immune component to help select patients who will benefit most from the therapies. “That means pathologists will be assessing not just the percent of tumor cells showing expression of PD-L1 but also the immune cell component, and that’s done by an area measurement. Whether the tumor component, immune component, or both need to be evaluated will depend on clinical trial results but will likely differ based on the tumor type.”
Given the complexity of this scoring, he sounds a note of caution for pathologists: Not all antibodies labeled PD-L1 stain the same. For example, three different assays reported at the IASLC annual meeting on the treatment of lung cancer with targeted therapies in February 2015 showed different levels of expression. (See Fig. 6.)
 The PD-L1 IHC assays may vary in their sensitivity, he says. In addition, they may differ in how they stain tumor cells versus immune cells. For example, one assay may stain tumor cells less intensely but the immune cells will appear much darker and easier to read, while another may stain tumor cells more strongly but the immune cells much more weakly.
The PD-L1 IHC assays may vary in their sensitivity, he says. In addition, they may differ in how they stain tumor cells versus immune cells. For example, one assay may stain tumor cells less intensely but the immune cells will appear much darker and easier to read, while another may stain tumor cells more strongly but the immune cells much more weakly.
Until there is better understanding of the reasons for these differences, “it is probably best to use the assays that were used in the clinical trials,” he suggests. For pembrolizumab, that means it’s best to use the pharmDX 22C3 assay, and for nivolumab it’s probably best to use the pharmDX 28-8 assay.
With the plethora of phase one and phase two drugs in the immuno-oncology pipeline just in the lung trials of one company, Bristol-Myers Squibb (see Fig. 7), the real promise of immunotherapy lies ahead, Dr. Bloom says. “There are a lot of ongoing trials and there are many more indications that are coming. Some might have testing, some might have no testing, and it’s conceivable that the cutoff could be different for each one.”
The message for pathologists is clear: “This is not going away. We are now moving beyond the tumor into the microenvironment, and we’re going to need to understand how all of these things interact with each other to move forward.”
Anne Paxton is a writer in Seattle. The immunotherapy webinar featuring Dr. Bloom, “Immune Checkpoint Blockade in Cancer,” is viewable at: https://j.mp/bloom_capwebinar. Dr. Bloom is a member of the CAP Personalized Health Care Committee.