Histologic features, differential diagnoses, unusual presentations
Karen Lusky
August 2022—The atypia in Epstein-Barr virus-positive mucocutaneous ulcers can mimic diffuse large B-cell lymphoma or classical Hodgkin lymphoma, a diagnostic pitfall that can result in overtreatment. And esophageal ulcers in immunocompromised patients should trigger cytomegalovirus immunohistochemistry in addition to GMS and herpes simplex virus-1 and -2 stains.
Those points and others were made in a CAP21 session on infectious disease of the gut, in which Alina Iuga, MD, Christina Wojewoda, MD, and Maryam Zenali, MD, presented cases to highlight the histologic features and differential diagnoses in an integrated anatomic and clinical pathology approach. Two of their cases follow. Three others were reported in the July issue (https://bit.ly/Gutbug-0722).
One of the cases presented is that of a 76-year-old woman with a clinical history of diarrhea, a remote history of ulcerative colitis, and a sigmoid ulcer on colonoscopy. The sigmoid biopsy seen in Fig. 1 shows colonic mucosa and several fragments of what appears at low power to be granulation tissue, said Dr. Iuga, director of gastrointestinal pathology and associate professor in the Department of Pathology and Laboratory Medicine at the University of North Carolina School of Medicine.
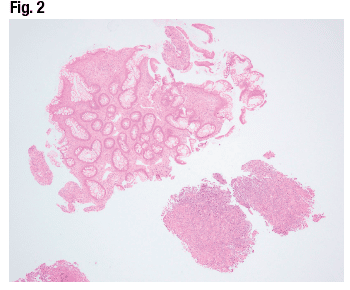
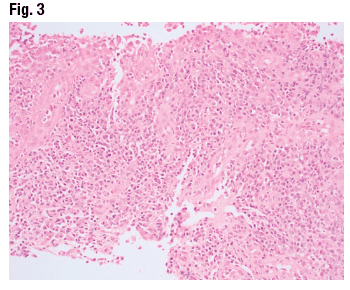
The adjacent colonic mucosa (Fig. 2) has mild glandular architecture distortion secondary to proximity to the ulcer, as well as mild lamina propria expansion by inflammatory infiltrate. A closer look at the ulcer bed (Fig. 3) reveals inflammatory infiltrate with a slight predominance of chronic inflammatory cells and cytologic atypia that prompted a hematopathology consult. The workup for lymphoma was negative.
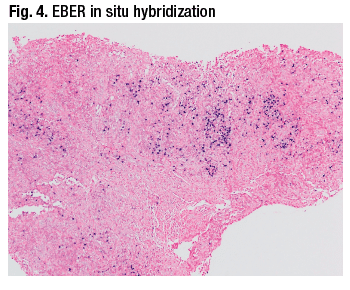
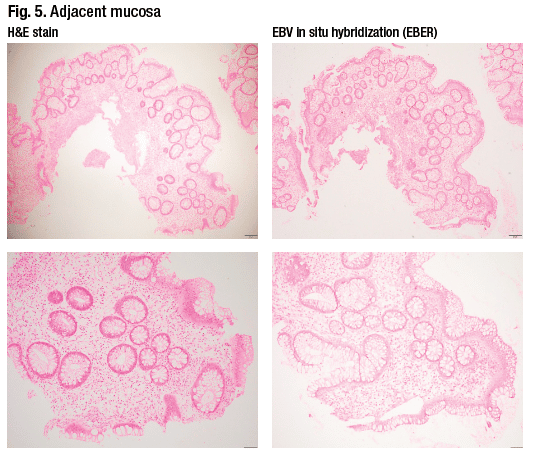
The differential diagnosis for this sigmoid ulcer includes inflammatory bowel disease, ischemia, drug effect, infection (e.g. CMV, EBV, lymphogranuloma venereum), and diverticular disease-associated colitis. In this case, Dr. Iuga said, the diagnosis was of an ulcer possibly associated with EBV infection because the Epstein-Barr encoding region (EBER) in situ hybridization stain showed numerous positive cells. The fragment seen in Fig. 4 shows a significant number of cells that are positive on EBER. The adjacent mucosa (Fig. 5) submitted separately shows mild architectural distortion suggestive of a chronic colitis, but no conspicuous staining on the in situ hybridization study for EBV.
In the microbiologic workup, serology is not useful in reactivation-type situations, said Dr. Wojewoda, director of microbiology at the University of Vermont Medical Center and associate professor, University of Vermont Larner College of Medicine. “The patients often are already EBV IgG positive, so we wouldn’t be able to tell if this was new infection or old infection,” she explained. “And EBV viral load or quantitative nucleic acid amplification could be helpful in these situations if it’s very high, although it’s often useful to know what the background level is.” As seen in this case, ISH on tissue was helpful. “It’s important to distinguish, if possible, between normal background levels of EBV and abnormally increased levels of EBV” that could suggest pathogenicity, Dr. Wojewoda said. “That’s why taking a look at the normal adjacent tissue with the EBER stain and comparing that to the lesional tissue with the EBV stain was important.”

The histologic findings combined with the viral stain are compatible with an EBV-positive mucocutaneous ulcer, Dr. Iuga said, which is “probably under-recognized in gastrointestinal pathology.” This ulcer, included in the latest WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, is typically a sharply circumscribed ulcer of the skin and oropharynx, “but can occur in the gastrointestinal tract in immunosuppressed patients on immunosuppressive therapy or due to advanced age” (Dojcinov SD, et al. Am J Surg Pathol. 2010;34[3]:405–417). “And the lesions can be single or multiple but usually are confined to an anatomic region.”
The EBV-positive mucocutaneous ulcer may show Hodgkin-like features. ”The lesions may show a polymorphous infiltrate and atypical large B-cell blasts with a Reed-Sternberg cell-like morphology. And these cells are positive for CD30 and EBER, and some even show reduced CD20 expression, in a background of abundant T cells.” Dojcinov, et al., reported that CD15 was positive in 43 percent of the 26 cases studied. Monoclonal TCR gene rearrangement was seen in 38 percent of cases, and B-cell clonality in 39 percent.
The atypia in the EBV mucocutaneous ulcer can mimic diffuse large B-cell lymphoma (monomorphic pattern) or classical Hodgkin lymphoma (polymorphic pattern), Dr. Iuga cautions. “This is a diagnostic pitfall that may result in overtreatment because, despite the presence of highly atypical cells in some of these lesions, the clinical course appears to be indolent and they don’t seem to progress to disseminated disease. There are even described cases with spontaneous remissions.”
EBV infection increases the risk of developing lymphoproliferative disorders in the GI tract, she said, including post-transplant lymphoproliferative disorder, Hodgkin and non-Hodgkin lymphomas, and lymphoepithelioma-like cancers and others, in particular gastric adenocarcinoma and cholangiocarcinoma.
In Fig. 6 is an example of a less common but under-recognized entity associated with EBV infection: an EBV-associated smooth muscle tumor, Dr. Iuga said. “These tumors can show a variable degree of cytological atypia. They may look like leiomyomas or leiomyosarcomas. They are positive for smooth muscle markers,” in this case for desmin. “And they show diffuse positivity on EBV in situ hybridization.”
EBV infection also might lead to development of a colitis resembling inflammatory bowel disease that presents with deep ulcers, persistent bloody diarrhea, and dense lymphoplasmacytic infiltrates. “And there is a chance that this EBV colitis can be misdiagnosed as inflammatory bowel disease. However, the issue is more complicated because inflammatory bowel disease also makes patients more prone to develop EBV infections due to immunosuppressive therapy.”
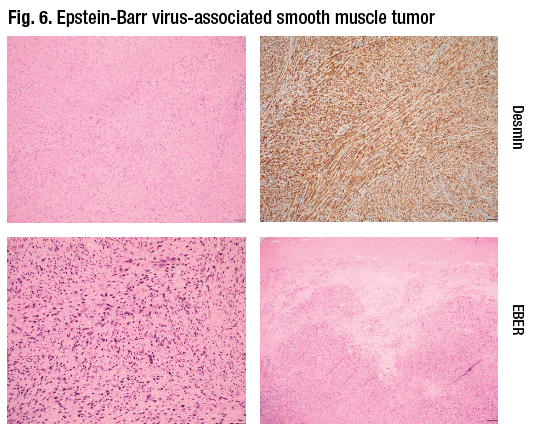
Fig. 7 is from the case of an 18-year-old with a first episode of GI bleeding and clinical suspicion for ulcerative colitis. “The H&E shows the classical features of a chronic active colitis,” Dr. Iuga said, “and EBV may highlight a couple of positive cells. However, it is probably not indicated to do the stain in any setting; one should perform the stain when there is a clinical suspicion for an EBV colitis or a persistent ulcer containing immune cells with cytologic atypia.” Random EBV in situ hybridization stains on colitis may highlight rare positive cells representing latent EBV or a local reactivation of EBV in inflamed mucosa.

EBV can increase disease exacerbation in IBD and complicates its clinical course by increasing severity, inducing refractoriness, or increasing the incidence of relapse. “There are reports suggesting that some patients may respond to intravenous ganciclovir.” Proposed mechanisms of autoimmunity include cross-reactive humoral immune responses to EBV antigens and infection-driven persistence of autoreactive B cells, Dr. Iuga explained.
The second case is that of a 45-year-old man with AIDS, low CD4 count, and poor antiretroviral compliance who presented with chest pain, dyspnea, cough, and fever. Chest CT revealed a dominant cavitary mass in the posterior right upper lobe, several smaller pulmonary cavitations, and proximal esophageal dilatation. Initial infectious studies, including acid-fast sputum smears, were negative. His cough worsened despite broad-spectrum antibiotics, and chest imaging noted disease progression. Three weeks later he developed dysphagia and odynophagia. Serum PCR for CMV was elevated. “And on the upper GI endoscopy,” Dr. Iuga said, “there was a five-centimeter longitudinal ulcer in the mid-esophagus.” Within the ulcer, there was a 1-mm fistulous opening.


The esophageal biopsies (Fig. 8) show squamous epithelium, several fragments of inflamed fibroconnective tissue, and tissue fragments lined by columnar epithelium. On higher power (Fig. 9) a brush border is seen, consistent with a respiratory-type mucosa that appears inflamed. “There are scattered neutrophils in the tissue and also cytomegalic cells with nuclear and cytoplasmic inclusions consistent with a CMV infection. The CMV immunostain confirms the histologic impression,” Dr. Iuga said (Fig. 10), “and the diagnosis is compatible with a tracheoesophageal fistula positive for CMV.”

Similar to EBV, Dr. Wojewoda said, CMV is usually diagnosed by serology but it is not useful in reactivation. “IgG antibodies can be produced several weeks after the initial infection and persist for life. CMV IgM can determine recent infection, but most of the time most people are infected early in life and we are looking at a reactivation. So confirmation of tissue-invasive CMV disease is best performed on histopathology.” Tissue-based PCR can also be performed.
CMV is an encapsulated, double-stranded DNA β-herpesvirus. “As opposed to EBV, which likes to go to B cells, CMV likes to go to polymorphonuclear neutrophils, T cells, vascular endothelium, and salivary glands,” Dr. Wojewoda said. “And it is the reactivation that we see in immunocompromised patients that can cause morbidity and mortality.”

GI manifestations of CMV infection include gastritis, esophagitis, duodenitis, colitis, and pancreatitis, Dr. Iuga said. “The active viral replication occurs in the epithelial cells, and viral particles are shed into the circulation and the gut lumen. And we usually see the cells that show cytomegalic changes in endothelial cells lining blood vessels.” The typical endoscopic appearance of CMV esophagitis includes sharply demarcated serpiginous ulcerations, sometimes coalescing to form giant ulcers. In immunocompromised patients, esophageal ulcer should trigger CMV IHC in addition to GMS and HSV-1 and -2 stains. “A significant number of patients who present with upper abdominal pain and dysphagia show CMV infection on immunohistochemistry,” Dr. Iuga noted.
Fig. 11 is an example of CMV gastritis, with cytomegalic cells in lamina propria. “Interestingly, CMV gastritis can present with epigastric pain, and there is a possible association with postural epigastric pain. Some of these patients have less pain when they are in a supine position, especially in post-transplant patients, which can suggest CMV gastritis,” she said.
In the lower GI tract, CMV most often affects the colon, and the mucosa may show ischemic-type changes on histology. On endoscopy, the findings are usually mild and patchy erythematous colonic mucosa with edema and subepithelial hemorrhage, Dr. Iuga said. Less common is discrete ulceration surrounded by unremarkable colonic mucosa, and sometimes the ulcers may display pseudomembranes. “The involvement in CMV colitis can be confined to the proximal colon.”
IHC may be pertinent to diagnosing CMV infection of the GI tract in patients with immunodeficiencies, such as common variable immunodeficiency, who are at risk for CMV infection. And a third of patients with steroid-refractory IBD colitis have CMV infection, Dr. Iuga said. CMV infection needs to be ruled out in bone marrow transplant patients who develop diarrhea and for whom there is clinical concern for graft versus host disease, “especially because CMV infection can also show on histology an increase in crypt epithelial apoptosis, which is what we look for in graft versus host disease biopsies. There are also reports of CMV-associated pseudotumors—often initially misdiagnosed as lymphoma—and also of intestinal perforation secondary to CMV infection, most commonly in the distal ileum.”
CMV disease more frequently complicates ulcerative colitis than Crohn’s disease. “One study estimated the prevalence of CMV positivity at 4.6 percent in patients with ulcerative colitis, and 0.8 percent in patients with Crohn’s disease. And it is helpful to evaluate for CMV in patients with inflammatory bowel disease refractory to immunosuppression because a number of them respond to adjusted immunosuppression and antiviral therapy,” Dr. Iuga said. “However, detection of the virus in IBD patients does not necessarily mean that CMV is causing the flare.”
Co-infection with other organisms may occur, she noted, especially in immunosuppressed patients. Fig. 12, page 20, is an example of co-infection with herpesvirus—“an esophageal ulcer biopsy that shows several CMV-positive cells, and on histology there are nuclear inclusions suggestive of herpes. This is confirmed on the herpes immunostain.”

The patient in this case, with an unusual presentation of CMV infection as a tracheoesophageal fistula, received valganciclovir for 21 days. The esophageal stent placed at the time of biopsy was removed 35 days post-placement, with resolution of the ulcer. The repeat barium esophagram showed no structural abnormalities, Dr. Iuga said.
Esophagorespiratory fistulas can be seen in HIV but are rare and more commonly associated with mycobacterial infections and other infectious etiologies, such as HSV, Candida, Nocardia, and, less likely, CMV infection. “It is good to keep in mind,” Dr. Iuga said, “that sometimes fistulae can be related to infectious organisms detected on histology.”
Karen Lusky is a writer in Brentwood, Tenn.