Charna Albert
January 2023—Guidelines for the diagnosis of von Willebrand disease, published in 2021, have raised questions about which von Willebrand factor activity assay laboratories should use. Each has its strengths and weaknesses, and the FDA has cleared or approved some, but not all, of the newer automated assays. In addition, not all the assays or guidelines use the same activity-to-antigen ratio cutoff to discriminate type 1 from type 2 von Willebrand disease, making it difficult to compare accuracy.
“That just begins the challenge,” said Marian Rollins-Raval, MD, MPH, associate professor of pathology at the University of New Mexico and medical director, TriCore Special Coagulation Laboratory, speaking in a CAP22 session about the five major types of von Willebrand factor (VWF) activity assays. Then, too, more clinical studies evaluating the newer assays and comparing them with the original ristocetin cofactor assays are needed, said co-presenter Neil Harris, MD, clinical professor of pathology, immunology, and laboratory medicine at the University of Florida. Drs. Rollins-Raval and Harris are members of the CAP Hemostasis and Thrombosis Committee.
When assessing a patient with a bleeding tendency for von Willebrand disease (VWD), Dr. Harris said, start with a CBC, “because it could be a thrombocytopenia.” Prothrombin time and partial prothrombin time should be measured because PT is not prolonged in VWD. “You’ll want to look at fibrinogen and possibly the thrombin time as well,” he said. If results of all are normal or there’s an isolated prolonged PTT, “you want to do the initial VWF screening assays,” which include VWF antigen, VWF activity, and factor VIII activity. If one or more of these tests are abnormal, the patient should be referred for selected specialized VWD studies, including von Willebrand multimeric analysis (James PD, et al. Blood Adv. 2021;5[1]:280–300; Nichols WL, et al. Haemophilia. 2008;14[2]:171–232).
The first VWF activity assay was the ristocetin cofactor assay. “The original tests were done by optical aggregometry and were really lab-developed tests using fixed reagent platelets,” Dr. Harris said. These assays had poor precision and a high coefficient of variation, he said, leading to low intraassay and interlaboratory reproducibility. “But the ristocetin cofactor assay became the gold standard simply because it was the first assay.” It has since been automated on some coagulation analyzers, he said, and those assays typically use a commercially available reagent containing lyophilized donor platelets and ristocetin. “If you have the correct coagulation analyzer, you can do it with a turbidimetric analysis and you can measure the platelet agglutination and compare it with calibrators and controls,” he said. The automated assay, which is FDA cleared, has better precision than the aggregometry assay, but the limit of detection is not substantially improved.
The overall algorithm for diagnosis of VWD laid out in the 2021 American Society of Hematology, International Society on Thrombosis and Haemostasis, National Hemophilia Foundation, and World Federation of Hemophilia guidelines begins with the VWF antigen, platelet-dependent VWF activity, and factor VIII activity (James PD, et al. Blood Adv. 2021;5[1]:280–300) (Fig. 1). If VWF levels are greater than 50 IU/dL, VWD can be ruled out, Dr. Harris said. “If it’s less than 30 percent, you’ve diagnosed von Willebrand disease, but the next step is the activity-to-antigen ratio. We typically do a platelet-dependent VWF activity—which up until now was the ristocetin cofactor activity—divided by the von Willebrand factor antigen.” (The algorithm says VWF level 30 to 50 for simplicity; this refers to VWF levels of 0.30 to 0.50 IU/mL, with the caveat that the lower limit of the normal range as determined by the local laboratory should be used if it is less than 0.50 IU/mL.)
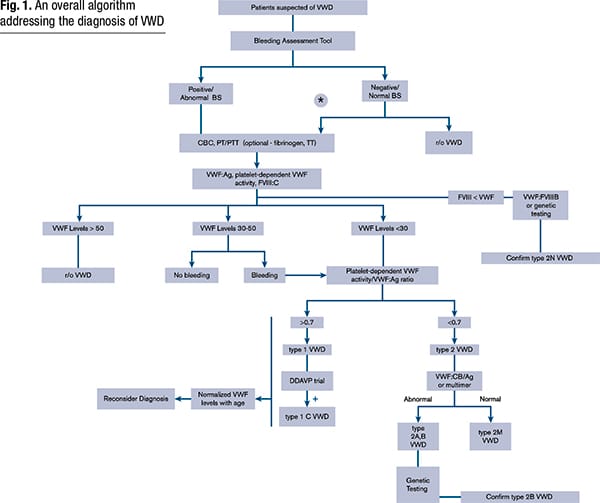
VWF levels refer to VWF antigen (VWF:Ag) and/or platelet-dependent VWF activity. The algorithm says VWF level 30 to 50 for simplicity; this refers to VWF levels of 0.30 to 0.50 IU/ml, with the caveat that the lower limit of the normal range as determined by the local laboratory should be used if it is <0.50 IU/ml. *Men and children, referred to a hematologist and/or first-degree relative affected with VWD. BS, bleeding score; CBC, complete blood count; DDAVP, desmopressin; FVIII, factor FVIII; FVlll:C, FVIII coagulant activity; PT, prothrombin time; PTT, partial thromboplastin time; r/o, rule out; TT, thrombin time; VWF:CB/Ag, ratio of VWF collagen binding to antigen; VWF:FVIIIB, VWF FVIII binding.
Reprinted from Blood Advances, vol 5(1), James P, Connell N, Ameer B, et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease, pages 280–300, Copyright 2021, with permission from Elsevier.
Per this new algorithm, a ratio of more than 0.7 classifies the more common type 1 VWD, caused by a partial quantitative deficiency of VWF. A ratio of less than 0.7 classifies the patient with type 2 disease, which encompasses the qualitative VWF defects and where “von Willebrand factor may be present in normal amounts, it may be reduced in some cases, but it’s lost some function,” Dr. Harris said. These are subclassified, most commonly, as types 2A, 2B, 2M, and 2N.

Dr. Harris
Those with intermediate levels of VWF activity (0.30–.50 IU/mL) are classified as “low VWF” if they have no bleeding, he said. “But if they have bleeding they would be put in the von Willebrand disease category.”
Type 2N, Dr. Harris said, is the functional variant in which “von Willebrand factor does everything in its normal capacity, except it lacks the ability to bind to factor VIII.” For patients with suspected type 2N in need of additional testing, the 2021 guidelines suggest using either VWF:factor VIII binding studies or targeted genetic testing for type 2N variants.
One of the problems with the ristocetin cofactor assay, Dr. Harris said, is the D1472H polymorphism, a common VWF ristocetin-binding polymorphism that reduces the apparent activity. When it’s present, “the ristocetin cofactor will give a low reading even though there are no clinical consequences,” he said. “It’s an in vitro effect, not an in vivo effect, and can lead to the overdiagnosis of von Willebrand disease.” The polymorphism (present in the A1 domain) is found in 63 percent of Blacks and 17 percent of the white population.
The guidelines suggest glycoprotein (GP) 1bM or GP1bR, newer automated assays that measure platelet-binding activity of VWF, over the automated or nonautomated ristocetin cofactor assay. “They call it a suggestion rather than a recommendation because the evidence wasn’t very certain,” Dr. Harris said. At the time the guidelines were published neither of these assays was FDA cleared, but one GP1bM assay received de novo FDA approval in 2022.
The GP1bR assay works as follows: In the presence of ristocetin, patient VWF binds recombinant wild-type GP1b attached by monoclonal antibodies to GP1b onto latex or magnetic beads. “If you add ristocetin and patient von Willebrand factor, the beads will agglutinate, and this can be monitored by an immunoturbidimetric or chemiluminescent assay.” The assay is not FDA cleared, though some laboratories have developed their own versions of both the GP1bR and GP1bM assays, he said.
“The GP1bM is a variation on the same idea,” Dr. Harris said. “You have a latex bead that’s coated with GP1b by virtue of a bridging antibody, except that the GP1b is a gain-of-function mutant which binds spontaneously to von Willebrand factor, to the A1 domain. So it’s exactly the same as the previous assay, except it doesn’t require ristocetin.” (The “R” in GP1bR stands for ristocetin, he noted, and the “M” in GP1bM stands for mutant.)
The VWF antibody assay is FDA cleared and gaining in popularity, Dr. Harris said. “When somebody first described this assay to me I said, ‘This can’t possibly work.’ But it does work,” he said. Without ristocetin, latex beads coated with monoclonal antibodies against the VWF A1 domain agglutinate with the addition of patient VWF. “I thought it wouldn’t work because I thought it would only pick up mutations in the A1 domain. But it was explained to me—and this was borne out by evidence—it also picks up defects in multimerization.” The antibody assay, which is available on some automated platforms, “is a great screen to discriminate patients with and without von Willebrand disease.”
With the antibody assay, additional VWF activity tests are likely to be needed for subclassification. “You certainly need the multimers and you may need collagen binding as well,” he said. The VWF antibody assay has similar sensitivity and specificity to the ristocetin cofactor assay, though it has comparatively increased precision and a decreased coefficient of variation at lower levels. It typically uses a VWF:Ab/VWF:Ag ratio of less than 0.7 to discriminate types 1 and 2, “though people have used higher ratios,” he said. “In our lab we run this assay. If the activity comes out low or less than 55 percent, we will reflex to the ristocetin cofactor assay.”
The collagen-binding activity assay, rather than measuring binding to platelets, measures binding to collagen. Magnetic particles are coated with a type III collagen triple-helical peptide. “You mix that in with patient plasma and the von Willebrand factor then binds onto the collagen, and that binding is detected by a labeled antibody to von Willebrand factor,” Dr. Harris said. The assay, which is not FDA cleared, is an automated chemiluminescent assay. Laboratory-developed ELISA-based assays also are available.
“Interestingly, the question has come up: Should we be doing collagen binding as well?” Dr. Harris asked. In type 2 von Willebrand disease, he said, collagen binding is decreased. And there may be two varieties of subtype 2M, he said: the collagen-binding variant and the GP1b variant. In the latter, VWF doesn’t bind to glycoprotein 1b, “which is what we usually think about with type 2M.” In this subtype the platelet binding activity-to-antigen ratio is decreased, but the collagen-binding-to-antigen ratio is normal. In the collagen-binding variant, this is reversed (Favarolo EJ, et al. Haemophilia. 2021;27[1]:137–148; Favarolo EJ, et al. Blood Adv. 2022;6[2]:416–419). Other studies have identified subtypes of the disease in which both platelet binding and collagen binding are decreased, he noted.
“So more studies are needed,” Dr. Harris said. “There’s a risk of bias in all studies due to case-control design.” In addition, the assays have been used to classify patients, as opposed to making a new diagnosis of VWD. And more FDA approvals are needed.
After the 2021 guidelines were published, the clinicians with whom Dr. Rollins-Raval works began to ask what they had never asked before: What VWF activity assay does the laboratory use? “So trying to justify the assay that we had and making sure it was a good assay for our patient population—that’s where I started down this rabbit hole.”
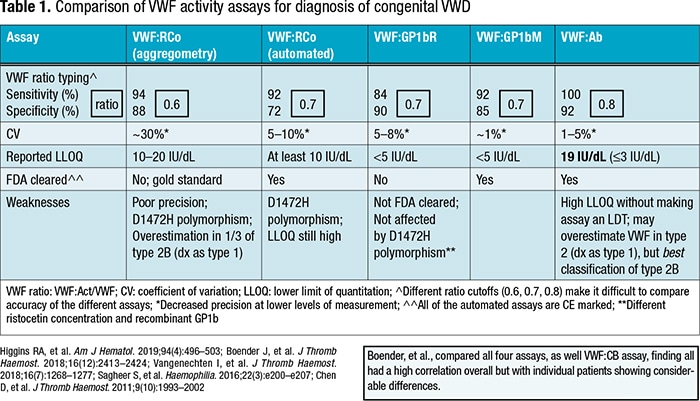
Dr. Rollins-Raval created a side-by-side comparison of five of the von Willebrand factor activity assays (excluding the collagen-binding assay) (Table 1). “The first thing I noticed was that we aren’t using the same ratios when we’re evaluating this,” she said. The sensitivity and specificity of the five assays are relatively similar, though the von Willebrand antibody activity appears superior in that category, “at least based on the study I reviewed and using that higher ratio [0.8].” As expected, she said, the manual ristocetin cofactor assay (aggregometry) has the highest coefficient of variation. “Automation has really helped with the precision of these assays, and that’s across the board,” she noted. With the aggregometry assay the lower limit of quantitation, too, was a challenge, “and the automated ristocetin did improve that some” but not as much as the other three assays have (GP1bR, GP1bM, Ab).
The manufacturer’s insert for the VWF Ab assay gives a lower limit of quantitation of 19 IU/dL, “which is a limitation,” Dr. Rollins-Raval said. “And if you lower that limit of quantitation, it does make it a laboratory-developed test.” The assay also may overestimate VWF in type 2, “so we might have slightly higher activity levels, which would then misdiagnose patients with type 1. But surprisingly, among these assays it appears to have the best classification of type 2B” (increased VWF affinity for GP1b with increased platelet clearance).
Though the major weakness with the ristocetin cofactor assays is their relatively poor precision, “there’s also the issue with both the automated and the aggregometry assays of over-detection or overdiagnosis” due to the D1472H polymorphism, she said. In addition, “with the aggregometry assay it does seem there’s an overestimation of the activity level in some studies of patients who have type 2B. So they would be mistakenly diagnosed as type 1 rather than type 2B, which could be an issue, especially with therapy.”
In some studies, the GP1bR assay has not been affected by the D1472H polymorphism. “And that was explained by the authors—they’re using a different ristocetin concentration in that assay, and they’re also using, instead of a native GP1b or a platelet GP1b, a recombinant GP1b. So that may explain why it’s not as affected by the polymorphism.”
Boender, et al., in 2018 compared the four assays and the VWF collagen-binding assay and found good overall correlation (Boender J, et al. J Thromb Haemost. 2018;16[12]:2413–2424). “But then if you look at individual patients there was considerable difference,” she said. “So you might need to look at your patient population to figure out which assay to choose.”

Dr. Rollins-Raval
A survey of laboratories that asked how reference intervals for VWF activity testing were established for the GP1bR, GP1bM, Ab, and ristocetin cofactor assays found that the majority of respondents did not use the manufacturer’s value. There was broad variability in how intervals were defined, with some taking a value from the literature, some from guidelines, and others determined in-house (Hollestelle MJ, et al. Semin Thromb Hemost. 2022;48[6]:739–749). “And even in this survey there was no discussion about how the VWF activity-to-antigen ratio was defined,” she said.
The current guidelines for VWF activity-to-antigen ratio verification suggest 0.7 as the cutoff to differentiate type 1 and type 2 von Willebrand disease, Dr. Rollins-Raval said. Though some still suggest a cutoff of 0.6, “even the FDA-cleared assays don’t validate this, because you’re dealing with two different assays.” And some labs may use an activity assay from one vendor and an antigen assay from another, or a lab-developed test for one and a commercial test for the other, “so it’s helpful to validate this ratio in your own lab with the reagents you’re using.”
Dr. Rollins-Raval’s laboratory performed a study to verify that the VWF activity-to-antigen and factor VIII-to-VWF activity ratios, using current reagents and the local population, were the same as the published guideline ratio cutoffs of 0.7. “We did limit it to 20 male and 20 female donors for measuring the VWF activity and the antigen and factor VIII activity,” she said. “And then we calculated the ratios for these and found the average and standard deviation for each ratio,” with the lower limit of each ratio range two standard deviations from the mean.
“Our acceptance criteria were that it must agree within 15 percent of that published cutoff for our lower limit ratio,” she continued. “Fortunately, it did work pretty well.” The VWF Ab activity-to-antigen ratio range was 0.7–1.0, and the factor VIII range was 0.7–1.3. “So we’re using 0.7 for both.” Testing a known bundle of VWD also would be helpful to verify that the 0.7 cutoff works for distinguishing type 1 disease from type 2, she noted.
In the September 2022 issue of Seminars in Thrombosis and Hemostasis are several articles on external quality assessment worldwide for VWF testing. One finding from a summary of the CAP’s proficiency testing, which looked at samples sent out for VWF antigen and activity testing between 2015 and 2020, is that the type of activity is changing over time (Salazar E, et al. Semin Thromb Hemost. 2022;48[6]:690–699).
“While in 2015 a lot of laboratories were doing the ristocetin cofactor assay [50 percent], that’s starting to decrease by 2020 [44 percent], and the VWF GP1b activity assays are starting to increase [28 percent and 45 percent, respectively],” Dr. Rollins-Raval said. “Interestingly, the coefficient of variation range is also starting to improve a little bit,” she said (14.6 to 44.8 percent in 2015, 8 to 34.8 percent in 2020). “It’s still not where we would like it, but, for coagulation, it is improving.” The survey also found that the automated ristocetin cofactor assay is now more common than the aggregometry assay, and the most commonly used GP1b assay is the VWF Ab assay (83 percent of participants).
More than 95 percent of participants who use the ristocetin cofactor assay and more than 89 percent of participants using the GP1b activity assays gave the correct qualitative interpretation (normal, type 1, or type 2), Dr. Rollins-Raval said. The GP1b activity means were consistently higher than the ristocetin cofactor means. “We’re not yet entirely sure what that means—this is a relatively limited study and some of these samples were manufactured, not patient samples,” she said. “But if you have a higher activity assay to antigen, you could be missing some of your type 2s. So that is something that we need to keep in mind in the laboratory.”
Charna Albert is CAP TODAY associate contributing editor.