Amy Carpenter Aquino
October 2023—Orders for cerebrospinal fluid testing for Alzheimer’s disease have grown at Mayo Clinic since spring 2020, when testing was first offered. When aducanumab was approved in May 2021, test orders jumped. And with lecanemab having received the Food and Drug Administration’s full approval on July 6, “we expect to see another leg up,” said Joshua Bornhorst, PhD, D(ABCC), speaking at the Association for Diagnostics and Laboratory Medicine meeting in July, where he reported Mayo’s experience with the Roche and Fujirebio assays.
“AD biomarkers can be implemented successfully in clinical laboratories,” said Dr. Bornhorst, consultant and assistant professor, Mayo Clinic Department of Laboratory Medicine and Pathology. “There are now FDA-approved options that generally show excellent precision, and there are instruments that are widely accessible.” The strict preanalytical protocols are a downside, and payment is still unresolved, though some insurers have it under consideration, he said.
“One shouldn’t base interpretation results on the ratios alone because comorbidities and other neurological disorders can affect the results of individual components,” said Dr. Bornhorst, who directs Mayo’s metals laboratory and co-directs the clinical immunoassay laboratory with Alicia Algeciras-Schimnich, PhD, professor of laboratory medicine and pathology, who was the scheduled presenter but unable to attend.
A workgroup of the National Institute on Aging and the Alzheimer’s Association presented in July at the Alzheimer’s Association International Conference a draft proposal for new Alzheimer’s disease diagnostic criteria. The criteria are a revision of the 2018 NIA-AA Alzheimer’s research framework and “further elucidate the potential role of biomarkers,” Dr. Bornhorst said. New to the draft revision is the incorporation of blood-based biomarkers.
Aβ42, one of the core CSF biomarkers, is decreased about 50 percent in Alzheimer’s disease due to the brain amyloid deposit.
“It’s thought that as these plaques form, it comes out of the CSF and can be measured in isolation, but more commonly it’s measured as a ratio”—Aβ42/Aβ40—“because Aβ40 is not incorporated as much and is not decreased,” Dr. Bornhorst said. “So it normalizes some of the measurements.”
Phosphorylated-tau represents increased tangle density, and several forms of p-tau are being evaluated, he said. Mayo Clinic uses p-tau181. “There’s more and more evidence that p-tau217 may be as or more effective in some cases, although the literature is mixed,” he said. Total-tau is a nonspecific general marker of neurodegradation and is included in some of Mayo Clinic’s panels. “NfL [neurofilament light chain] is coming on as a nonspecific marker of neurodegradation as well and has been incorporated in some clinical trials in recent studies of Alzheimer’s disease.” Total-tau and p-tau both rise to about 200 percent relative to concentrations found in the CSF of non-AD-affected individuals.
In the NIA-AA’s A/T/N biomarker classification system for Alzheimer’s disease, A stands for amyloid pathology and is measured by amyloid PET or CSF biomarkers Aβ42 or Aβ42/Aβ40. T is tangle pathology, which is assessed by tau-PET or CSF p-tau. N is neurodegeneration or neuronal injury, detected by 18F-FDG PET, structural MRI, or CSF t-tau. Other nonspecific markers of neuronal injury such as NfL are emerging.
Based on the A/T/N biomarker combinations and whether each marker is negative or positive, there are eight commonly seen biomarker profiles. The four on the Alzheimer’s continuum are as follows: A+T−(N)−: Alzheimer’s pathologic change; A+T+(N)−: Alzheimer’s disease; A+T+(N)+: Alzheimer’s disease; and A+T−(N)+: Alzheimer’s and concomitant suspected non-Alzheimer’s pathologic change. Three profiles in which there may be an elevated N or T marker without a corresponding decrease in amyloid “may be representative of non-Alzheimer’s pathologic change and may point the clinician in a different direction,” Dr. Bornhorst said. Normal Alzheimer’s disease biomarkers are A−T−(N)−.
Mayo Clinic has offered CSF biomarker testing for Alzheimer’s disease evaluation since March 2020, when the laboratory launched its automated Elecsys Gen 1 panel consisting of Aβ42, t-tau, and p-tau181.
Low-bind polypropylene tubes are used to collect CSF and provided by the laboratory. They reduce the amyloid deposition that can occur upon collection (Van Harten AC, et al. Alzheimers Dement. 2022;18[4]:635–644).
The test is a laboratory-developed procedure, Dr. Bornhorst said. “We based cutoffs on concordance to amyloid PET based on an in-house study. At Mayo, we’re fortunate to have the Mayo [Clinic Study of] Aging cohort, from which we had a large number of samples from people who had been neurologically evaluated and diagnosed with Alzheimer’s or non-Alzheimer’s.”
Mayo Clinic began last year to also offer the FDA-cleared Fujirebio Lumipulse G β-Amyloid Ratio (1–42/1–40) in vitro diagnostic test.
The p-tau/Aβ42 ratio cutoff point of less than or equal to 0.023 is the important number for the Roche Gen 1 assays, Dr. Bornhorst said. “It provides the optimal balance between negative percent agreement and positive percent agreement when compared to amyloid PET results.” The normal cutoff point for Aβ42 is greater than 1026 pg/mL, for p-tau181 less than or equal to 21.7 pg/mL, and for t-tau less than or equal to 238 pg/mL. “With our population, we’ve got about a 92 percent concordance with both positive and negative with amyloid PET,” he said. Important to note, he cautions, is that these cutoff values can change and have changed with assay modification and restandardization.

Dr. Bornhorst
The Alzheimer’s Association consensus protocol recommendations call for a lumbar puncture, discarding the first 1 to 2 mL of CSF, and using a gravity-drip method for CSF collection directly into a low-bind polypropylene tube. The syringe-pull method increases collection speed, but the drip method exposes the sample to less manipulation, metal, and plastic and reduces the risk of Aβ42 binding to the plastic of the syringe, which could affect the ratio. The Sarstedt CSF false-bottom tube 63.614.625 (2.5 mL) is preferred.
Internal studies revealed that if the collection tube is at least 50 percent full, there is little volume-to-tube-surface effect, Dr. Bornhorst said. “We put footnotes on tubes that are less than 50 percent full” because low sample volume can affect the overall interpretation.
Compliance with collection tube requirements in 2020 was at about 60 percent. “Any laboratory offering these tests, I believe, will have that compliance challenge,” he said. Footnotes and client education raised compliance to its rate this year of about 90 percent.
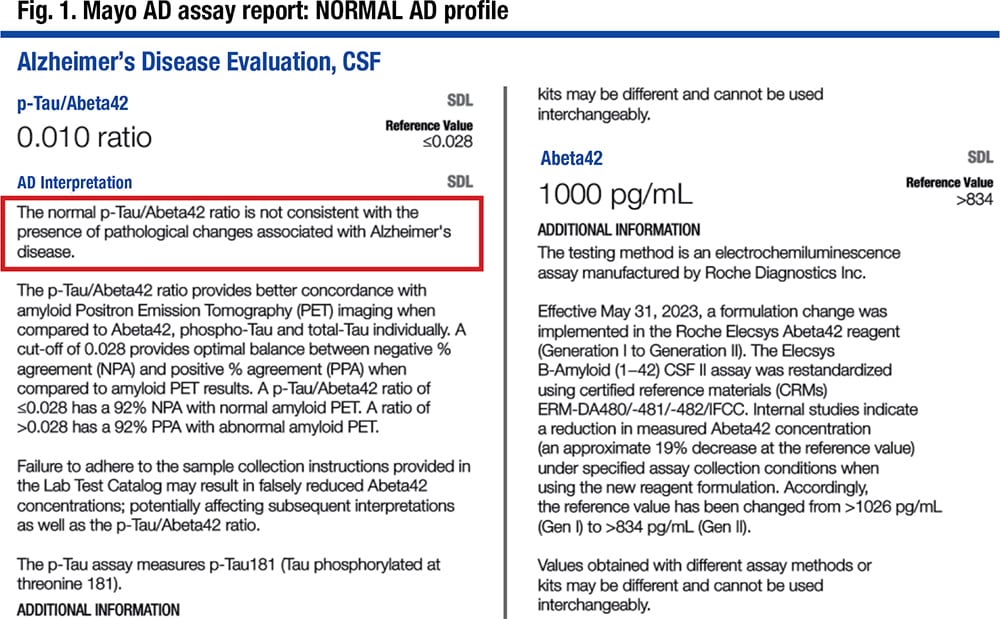
In Fig. 1 is a laboratory report. “The important value is the p-tau/Aβ42 value,” Dr. Bornhorst said, noting it is 0.010 and reported as a normal profile, not consistent with the presence of pathological changes associated with Alzheimer’s disease. The components are reported quantitatively but without interpretation. An elevated t-tau level may point to other neurological diagnoses.
Dr. Bornhorst reported one year of the laboratory’s experience with the Elecsys Gen 1 assay.
About 25 percent of results were all normal, 25 percent all abnormal, and 40 to 50 percent were mixed ratios. “Because the ratio is diagnostically better and has been established at many points in individual studies, we feel that we make the abnormal/normal call based on the ratio, even if all the markers are not abnormal, and that’s been borne out in our concordance studies to the presence of amyloid PET,” he said.
One population of special interest are those whose samples are normal for p-tau, t-tau, and p-tau/Aβ42 but abnormally low for Aβ42 only. The frequency is 19 percent for the Mayo Clinic laboratory and 16 percent for the reference laboratory. “Our thinking initially was that this is perhaps a result of preanalytic problems”—adhesion or absorption into the tubes, less volume, or inappropriate collection, he said. But a study of the data revealed “a different pattern that could point us either way.”
They therefore undertook a study of 535 patients who had Elecsys CSF biomarker testing over a one-year period. A neurologist who was blinded to imaging and CSF test results retrospectively assigned a clinical diagnosis. Associations between the blinded clinical diagnoses and CSF biomarkers were carried out via a Chi-squared test of independence.
Isolated abnormal Aβ42 is nonspecific, Dr. Bornhorst said. “There is the problem of preanalytical issues, but there is also something called normal pressure hydrocephalus.” It is a rare disorder, but in that patient cohort, 52 percent had an abnormal NPH. “In that disorder you get an increase of CSF volume,” he said, “and we think that drives down the overall Aβ42 concentrations” (Li W, et al. Presented at: American Academy of Neurology Annual Meeting; April 2–7, 2022; Seattle. S2.005). This points to a need for reporting quantitative markers rather than just the resulting ratio, he said.
Most of the patients with other clinical diagnoses, such as AD-related dementia, had an abnormal ratio, and a few also had an abnormal Aβ42 level. “So we did association studies,” he said, and “an abnormal ratio was strongly associated with AD dementia.”
“NPH had a very strong association with Aβ42 being the only discrepant or abnormal marker.”
Mayo launched in May 2022 the Fujirebio Lumipulse G β-Amyloid Ratio (1–42/1–40) in vitro diagnostic test as an FDA-approved assay for use in patients age 55 and older.
When the FDA-cleared Roche ratio became available in December 2022, which was the ratio Mayo had been using in the Gen 1 assay, a recalibration was needed because of the change from Gen 1 to Gen 2. The new Gen 2 assays were slightly different and resulted in a slightly different cutoff ratio for the Elecsys assays Mayo had been using in order to maintain the previously validated clinical performance of the assay.
The FDA indicated that for each company’s assay, the laboratory can report only the ratio, in patients age 55 and older, and as a positive or negative result, or likely positive in the case of the Fujirebio assay.
The Mayo Clinic laboratory team compared the two ratios—p-tau/Aβ42 (Gen 1) and Aβ42/40—in a study of 150 individuals and found them to show similar agreement with amyloid PET (Campbell MR, et al. Alzheimers Dement. 2021;13[1]:e12190). “We had 97 percent concordance between the [Lumipulse] Aβ42/Aβ40 ratio and the [Roche] p-tau/Aβ42 ratio,” Dr. Bornhorst said, and “when we expanded that, they both showed equivalent performance with amyloid PET.”
Moving to the FDA-cleared Roche p-tau/Aβ42 ratio raised several considerations, one of which was the change from Gen 1 to Gen 2. The others were the more restrictive predefined collection protocol, a restandardization of the Aβ42 assay with an approximately 19 percent decrease at the reference value, and an increased biotin interference threshold for Aβ42 and p-tau.
Mayo implemented the Roche Elecsys Gen 2 assays in June but retained the laboratory-developed test classification because of the age cutoff, “which we felt was restrictive,” he said, and because they felt their existing preanalytic protocols were working well. In addition, Mayo neurologists wanted the quantitative results, “which was prohibited by FDA in an FDA-approved assay.”
“In our population,” he said, “we adjusted the cutoffs based on the method comparison between Gen 1 and Gen 2 back to our original studies to maintain what we felt was equivalent diagnostic performance” (Fig. 1).
The three methods used to evaluate patients with cognitive decline for Alzheimer’s disease vary in performance.
Clinical diagnosis has a sensitivity of about 80 percent and a specificity of about 70 percent, Dr. Bornhorst said. Amyloid PET sensitivity is at 90 percent; specificity is at 84 percent. While CSF biomarkers require a collection technique many patients would rather avoid, the biomarkers have a sensitivity of about 92 to 96 percent and a specificity of about 88 to 90 percent.
“All three play a role in the establishment of Alzheimer’s disease,” Dr. Bornhorst said. No method is used in isolation, and clinical evaluation remains important regardless of the diagnostic methods used in conjunction.
CSF biomarker testing “is not meant for screening,” or for patients not considered to be at risk for AD, he said. Symptoms of REM sleep behavior disorder are not reason to test, he said, nor is a determination of disease severity in patients already diagnosed with AD. “That may be changing off-label. We’ll see how this develops as drugs come on the scene and treatment monitoring progresses.”
Other inappropriate uses: in those who are apolipoprotein E 4 carriers with no cognitive impairment, in lieu of genotyping for suspected autosomal dominant AD, and in autosomal dominant AD mutation carriers, with or without symptoms.
“Additional work needs to be done in the real world to further characterize the performance in retrospective and prospective clinical cohorts of patients with mild cognitive impairment,” Dr. Bornhorst said.
Amy Carpenter Aquino is CAP TODAY senior editor.
Add Custom Script