Zaibo Li, MD, PhD
Abha Goyal, MD
Eric Huang, MD, PhD
May 2023—Standardized reporting systems have been developed during the past decade for cytopathology of different organ systems including the pancreaticobiliary system. The Papanicolaou Society of Cytopathology (PSC) in 2014 published the first reporting system for pancreaticobiliary cytology.1 Studies have demonstrated that implementation of the PSC reporting system has significantly reduced the number of “atypical” interpretations and increased the number of specific diagnoses.2-8
Recently, the World Health Organization, International Academy of Cytology, and International Agency for Research on Cancer presented a standardized reporting of pancreaticobiliary cytopathology with a seven-tiered system by revising the PSC system for reporting pancreaticobiliary cytology.9 This reporting system is one of the first in a series from various body sites that mirror the WHO Classification of Tumors series and provides an evidence-based terminology system with associated risk of malignancy and diagnostic management recommendation per diagnostic category. The WHO Reporting System for Pancreaticobiliary Cytopathology revises the PSC system and replaces the six-tiered system with a seven-tiered system including “insufficient/inadequate/nondiagnostic,” “benign/negative for malignancy,” “atypical,” “pancreaticobiliary neoplasm-low risk/low grade (PaN-Low),” “pancreaticobiliary neoplasm-high risk/high grade (PaN-High),” “suspicious for malignancy,” and “positive for malignancy” (Table 1).
Insufficient/inadequate/nondiagnostic. A pancreaticobiliary cytology specimen is categorized as insufficient/inadequate/nondiagnostic if it does not permit a diagnosis of the targeted lesion for qualitative and/or quantitative reasons, such as obscuring artifact, gastrointestinal epithelium only, normal elements only in the setting of a clearly defined solid or cystic mass, acellular aspirate of a solid mass, or a cystic mass without evidence of mucinous etiology (i.e. elevated CEA, thick mucin, or presence of KRAS/GNAS mutation). These may be caused by technical, preparation, or sampling issues. There is no minimal number of cells to define this category, but any atypia precludes use of this category.
The average frequency of this category is about 12 percent and the risk of malignancy (ROM) ranges from five percent to 25 percent (higher in bile duct strictures: 28 percent to 69 percent). It is necessary to correlate with clinical information and imaging before providing this interpretation, and it is also important to check the biochemical analysis results in fluids/cyst content for cystic lesions. Repeating aspiration is recommended for patients with this interpretation, and employing rapid onsite evaluation (ROSE) or a different method may be helpful.
Benign/negative for malignancy (non-neoplastic and neoplastic). A pancreaticobiliary cytology specimen is categorized as benign or negative for malignancy if it demonstrates unequivocal benign cytopathological features, which may or may not be diagnostic of a specific process or a benign neoplasm. It should be adequately cellular with no sign of atypia. It may contain only normal pancreatic ductal or acinar cells in the context of a vaguely formed lesion without a distinct mass. Non-neoplastic lesions in this category include benign pancreatic tissue with a vague mass on imaging, inflammatory processes, pseudocyst, lymphoepithelial cyst, and accessory spleen. Benign neoplasms in this category include serous cystadenoma, schwannoma, lymphangioma, and other rare benign neoplasms.
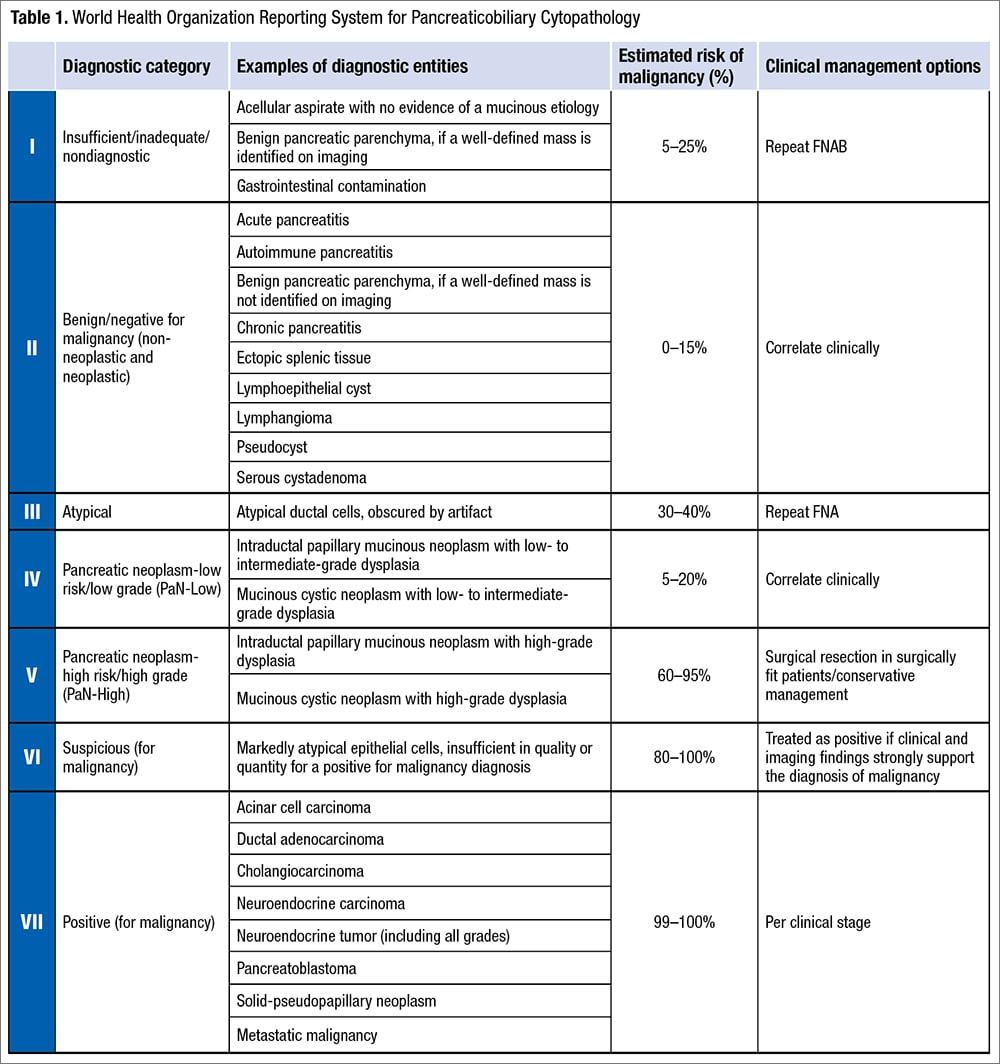
The frequency of this category is about 15 percent and the ROM ranges from zero to 15 percent (higher for bile duct lesions, up to 55 percent). Surgical management usually is not necessary for patients with this interpretation except for large serous cystadenomas.
Atypical. A pancreaticobiliary cytology specimen is categorized as atypical if it demonstrates features that may raise the possibility of a malignant lesion but are insufficient either in number or in quality for the diagnosis of a benign, PaN-Low, PaN-High, or malignant lesion. This interpretation may be due to low cellularity, poor preservation, technical issues, lack of availability of ROSE, or a high threshold for a malignant diagnosis (which is more common with biliary brushings).
The frequency of the atypical category is about 5.5 percent (range: zero percent to 14 percent) in pancreatic fine-needle aspirations. The frequency is higher in bile duct brushings (range: 11 percent to 39.8 percent), resulting from reactive atypia inherent to primary sclerosing cholangitis, stents or biliary stones, and difficulties encountered in the interpretation of well-differentiated adenocarcinomas. ROM of the atypical category is 30 to 40 percent for pancreatic FNAs and 25 to 61 percent for bile duct brushings. Management of patients with atypical interpretation includes consensus review, expert consultation, use of ancillary tests (FISH, molecular testing with next-generation sequencing) to further refine the diagnosis, multidisciplinary discussion, or repeat sampling.
Pancreaticobiliary neoplasm-low risk/low grade (PaN-Low) and high risk/high grade (PaN-High). One of the major changes in the WHO system is the replacement of the original PSC category of “neoplastic: other” by two distinct categories: PaN-Low and PaN-High. This dichotomization was prompted by the inclusion of low-grade and high-grade entities with a widely disparate ROM (14 to 100 percent) together in the PSC system. These two categories of the WHO system comprise the low-grade and high-grade forms of intraductal and/or cystic neoplasms of the pancreaticobiliary tract respectively, i.e. intraductal papillary mucinous neoplasm (IPMN), mucinous cystic neoplasm (MCN), pancreatic intraepithelial neoplasia, biliary intraepithelial neoplasia, and intraductal papillary neoplasm of bile ducts. The high-grade category also includes intraductal oncocytic papillary neoplasm and intraductal tubulopapillary neoplasm.

The low-grade pancreatic mucinous neoplastic cysts—i.e. IPMNs and MCNs with low- to intermediate-grade dysplasia—mostly reveal low cellularity. The background may show colloid-like thick mucin, which aids in interpreting a neoplastic mucinous cyst. The epithelium itself is composed of columnar cells, with size similar to that of a duodenal enterocyte, that are arranged in evenly spaced, mildly crowded, or pseudostratified groups. They have moderate to abundant mucinous cytoplasm, smooth to mildly irregular nuclear membranes, even chromatin, and they may harbor intranuclear inclusions. The main differential diagnosis includes gastrointestinal tract contaminant epithelium. Ancillary tests including a carcinoembryonic antigen level of greater than 192 ng/mL and presence of KRAS/GNAS/RNF43 mutations can aid significantly in the diagnosis of a neoplastic mucinous cyst.
High-grade neoplastic mucinous cysts of the pancreas mostly include IPMNs and MCNs with high-grade dysplasia or even an associated invasive carcinoma. High-grade atypia is typically seen as clusters of or singly lying cells smaller than a duodenal enterocyte (< 12 µm) with variably mucinous cytoplasm, high nuclear-cytoplasmic ratio, hyperchromasia or hypochromasia, and irregular nuclear membranes. The background may reveal necrosis. Ancillary studies can be employed to assess the grade of the cyst. Mutations in TP53, SMAD4, CDKN2A, PTEN, or PIK3CA can be associated with high-grade cysts. Immunohistochemical staining on cell block sections with p53 (strong and diffuse or null pattern) or SMAD4 (loss of staining) can aid in the diagnosis as well.
The ROM for the PaN-Low category has been reported to be five to 20 percent (with no data yet for bile duct brushings). Management may involve clinical correlation with repeat sampling in conjunction with ancillary studies (i.e. FISH/NGS). For the PaN-High category, the ROM ranges from 60 to 95 percent. Surgical resection is appropriate in surgically fit patients; however, conservative follow-up may be considered if there are increased risks involved with surgery or the imaging is not high risk.
Suspicious for malignancy. Suspicious for malignancy is used when a specimen demonstrates cytologic features worrisome for malignancy but insufficient in quantity or quality to make a definitive diagnosis of malignancy. This category accounts for about five to 16 percent of the pancreaticobiliary tract cytologic diagnoses reported in the literature. Reasons for using this category include sparse cellularity, poor preservation or staining quality, or concurrent pancreatitis, stents, stones, and inflammatory conditions. The ROM for suspicious for malignancy in pancreatic FNA and bile duct brushing ranges from 80 to 100 percent and 74 to 100 percent, respectively, reflecting the degree of interobserver variability.
When using the suspicious category, a detailed note should be included in the report to convey the worrisome cytologic features in the sample and why a definitive “malignant” diagnosis is not made. The results of ancillary testing (if available) with correlation to clinical and radiologic features should be emphasized when managing these patients. Suspicious for malignancy should not be treated the same as malignant diagnosis. It is best to discuss these patients in multidisciplinary conference where all the relevant information can be gathered. However, if clinical information and imaging strongly support a malignancy, definitive therapy can proceed without additional tissue confirmation.
Positive for malignancy. A malignant diagnosis is provided when there are unequivocal cytologic features of malignancy. The malignant cytologic features for pancreatic ductal adenocarcinoma include hypercellular sample, loss of the normal honeycomb architecture, enlarged nuclei, vesicular chromatin, irregular nuclear contours, size variation of 4:1 in a given fragment, and atypical mitoses. The malignant category includes primary and metastatic diseases. In contrast to the PSC system, well-differentiated pancreatic neuroendocrine tumors (PanNET) and solid pseudopapillary neoplasms (SPN) are included in the new WHO classification as malignant to align with the WHO Classification of Tumours of the Digestive System. The ROM for malignant category in pancreatic FNA and bile duct brushing ranges from 99 to 100 percent and 96 to 100 percent, respectively. Other entities in this category include acinar cell carcinoma, cholangiocarcinoma, and pancreatoblastoma.
Malignant diagnosis typically will be managed with surgical excision for primary pancreaticobiliary tract diseases. However, patients with PanNET smaller than 2 cm and Ki-67 less than three percent may be managed with surveillance. More advanced diseases may be treated with chemotherapy and radiation therapy.
Comparison between WHO and PSC systems (Table 2). One major change in the WHO system, as compared with the PSC system, is the classification of neoplasia. In the PSC system, there is a single category for neoplasms including “benign” (serous cystadenoma and lymphangioma) and “other” (IPMNs of any grade, MCNs of any grade, PanNETs, and SPNs). In the WHO system, these neoplasms are classified into four categories: benign/negative for malignancy (serous cystadenoma and lymphangioma), PaN-Low (IPMN/MCN with low- to intermediate-grade dysplasia), PaN-High (IPMN/MCN with high-grade dysplasia), and positive for malignancy (PanNETs and SPNs). Additionally, pancreatic FNAs with findings suspicious for a well-differentiated PanNET or SPN that are categorized as atypical in the PSC system are now reclassified as suspicious for malignancy per the WHO system recommendation.
Summary. The WHO Reporting System for Pancreaticobiliary Cytopathology mirrors the WHO Classification of Tumors series and provides an evidence-based terminology system with associated ROMs to assist the clinical care team in managing the patient. The performance indicators (ROM) for each category of the WHO system were derived from literature based on the previous system, and therefore future studies focusing on the current WHO system are warranted.
- Pitman MB, Layfield LJ. The Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology: Definitions, Criteria and Explanatory Notes. Springer; 2015.
- Layfield LJ, Dodd L, Factor R, Schmidt RL. Malignancy risk associated with diagnostic categories defined by the Papanicolaou Society of Cytopathology pancreaticobiliary guidelines. Cancer Cytopathol. 2014;122(6):420–427.
- Smith AL, Abdul-Karim FW, Goyal A. Cytologic categorization of pancreatic neoplastic mucinous cysts with an assessment of the risk of malignancy: a retrospective study based on the Papanicolaou Society of Cytopathology guidelines. Cancer Cytopathol. 2016;124(4):285–293.
- McKinley M, Newman M. Observations on the application of the Papanicolaou Society of Cytopathology standardised terminology and nomenclature for pancreaticobiliary cytology. Pathology. 2016;48(4):353–356.
- Hoda RS, Finer EB, Arpin RN III, Rosenbaum M, Pitman MB. Risk of malignancy in the categories of the Papanicolaou Society of Cytopathology system for reporting pancreaticobiliary cytology. J Am Soc Cytopathol. 2019;8(3):120–127.
- Sung S, Del Portillo A, Gonda TA, Kluger MD, Tiscornia-Wasserman PG. Update on risk stratification in the Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology categories: 3-year, prospective, single-institution experience. Cancer Cytopathol. 2020;128(1):29–35.
- Gilani SM, Adeniran AJ, Cai G. Endoscopic ultrasound-guided fine needle aspiration cytologic evaluation of intraductal papillary mucinous neoplasm and mucinous cystic neoplasms of pancreas. Am J Clin Pathol. 2020;154(4):559–770.
- Ozretic´ L, Simonovic´ AV, Rathbone ML, Young MPA, Perez-Machado MA. The benefits of the Papanicolaou Society of Cytopathology System for reporting pancreatobiliary cytology: a 2-year review from a single academic institution. Cytopathology. 2021;32(2):227–232.
- Pitman MB, Centeno BA, Reid MD, et al. The World Health Organization Reporting System for Pancreaticobiliary Cytopathology. Acta Cytol. Published online Dec. 14, 2022. doi:10.1159/000527912.
Dr. Li is an associate professor of pathology at Ohio State University Wexner Medical Center, Dr. Goyal is an associate professor of clinical pathology and laboratory medicine at Weill Cornell Medicine, and Dr. Huang is an associate professor of laboratory medicine and pathology at the University of Washington. Drs. Goyal and Huang are members of the CAP Cytopathology Committee and Dr. Li is a former member of the committee.