Rohit Sharma, MD
Zhiqiang Wang, MD, PhD
 October 2016—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the University of Florida College of Medicine, Jacksonville. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
October 2016—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the University of Florida College of Medicine, Jacksonville. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Molecular analysis of tumor cells is a multistep process dependent on many factors. A key step in that process is the isolation of tumor cells by means of microdissection techniques. The goal in the dissection is to isolate the tumor cells with minimal contamination of surrounding and admixing non-neoplastic cells to avoid masking of tumor-specific alterations. Several techniques can be used for this purpose, including manual tissue dissection, which is suitable for larger cell populations with relatively high neoplastic proportion against non-neoplastic cells, and more precise techniques such as laser capture microdissection (LCM), which is suitable for smaller neoplastic populations.<sup>1,2 </sup> Each technique has advantages and disadvantages that must be taken into consideration. The following case illustrates how LCM plays an important role in isolating tumor cells and allowing for detection of a mutation with therapeutic implications for the patient.
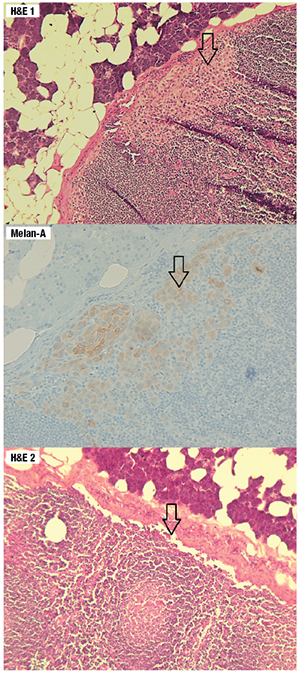
Fig. 1. The top and middle graphics reveal a focus of micrometastasis that is positive for Melan-A. The bottom graphic is the final H&E cut from the same tissue block which shows absence of the micrometastasis, demonstrating paucity of the tumor load.
Case presentation. A 40-year-old man with a family history of malignant melanoma in his grandmother and aunt presented with a nonresolving cutaneous crusting lesion in the right preauricular region. An excisional biopsy was performed at an outside institution which revealed a malignant melanoma (depth: 2.8 cm, positive ulceration, 4 mitoses/mm2, indeterminant lymphovascular space invasion, no apparent neural invasion) with clear margins (closest 2 mm anterior margin).
He also presented with two additional pigmented lesions, one from his thigh and another from his left lower abdominal quadrant, both of which were negative for melanoma. Atypical melanocytic proliferation, however, was present within one of the lesions. He underwent imaging with brain MRI and CT scan of the chest, abdomen, and pelvis, which were negative for metastatic disease. A subcentimeter hypodensity in the liver was identified, too small to characterize but consistent with a cyst. He further went on to undergo a positron emission tomography and computed tomography scan, which by the patient’s report was negative although the imaging and official report were not available.
Since the preauricular lesion was nonresolving, he underwent a right parotidectomy and right neck dissection which revealed five out of 12 intra-parotid and peri-parotid lymph nodes that were positive for micrometastasis. No residual primary melanoma was identified. The largest metastatic focus was seen in the subcapsular space of a 2.2-mm intra-parotid lymph node measuring 1.0 × 0.2 mm in dimension. The metastatic focus was absent on the final H&E stain after processing for Melan-A immunohistochemistry and three consecutive cuts at 8 µm for microdissection.
Given that the primary tumor was excised at an outside institution and the tissue block was not available, DNA recovery from the micrometastatic tumor foci were attempted. To conduct LCM, unstained paraffin-embedded sections were stained using the Arcturus Paradise Stain (Thermo Fisher). LCM was then carried out with the Arcturus PixCell II microscopic system to capture the target tissue to Arcturus CapSure Macro LCM Caps for DNA extraction by Arcturus PicoPure DNA Extraction Kit following the manufacturer’s instructions. DNA was quantitated by NanoDrop 2000 spectrophotometer (Thermo Scientific) and PCR amplified, followed by pyrosequencing. Fig. 1 (page 44) shows subcapsular micrometastasis with H&E stains and the immunohistochemical stain for Melan-A in one of the positive lymph nodes with the highest tumor load. Fig. 2 illustrates the results before and after the laser microdissection. Fig. 3 depicts the results of pyrosequencing analysis that indicate the presence of BRAF c.1799T>A, p.Val600Asp mutation.
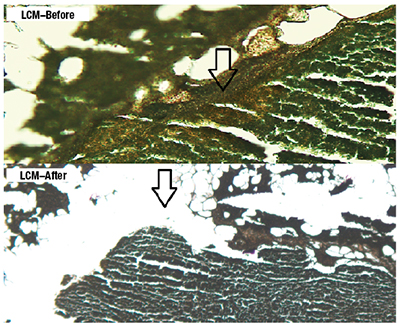
Fig. 2. Laser capture microdissection was performed on the slides, which were cut prior to the final H&E stain. This graphic shows the slides that were stained using the Arcturus Paradise Stain (Thermo Fisher) and the results of before and after the laser dissection process.
Discussion. Malignant melanoma is a very aggressive entity with limited therapeutic choices and an unfavorable prognosis. Targeted therapy with vemurafenib, dabrafenib, and trametinib has provided additional therapeutic options for malignant melanoma patients.3 However, use of this particular therapy is dependent on the molecular companion test to identify the mutations these drugs work against, which in this case is in the BRAF gene. The BRAF c.1799T>A, p.Val600Asp mutation, however, is not restricted to melanoma; it is seen in other solid tumors—for example, thyroid, lung, and colorectal cancers.4–6 In addition to serving as a companion test for actionable targeted therapy of melanoma and probably non-small cell lung carcinoma, the BRAF c.1799T>A, p.Val600Asp assay also finds its role in diagnostics (papillary thyroid carcinoma, reflex test in MSI-H colorectal carcinoma for Lynch syndrome) and prognostics (colorectal carcinoma).
The entire process in identifying the mutation is dependent on achieving a good sample. This can be accomplished through the precise isolation of the tumor cells from histological sections. These tumor cells may be in small groups or even single cells that admix with normal tissue. The worst-case scenario lies with micrometastasis in a lymph node. The high wild type DNA content from the background admixing lymphocytes increases the difficulty of this process. This is where the value of techniques like LCM lies compared with other techniques such as manual tissue microdissection.
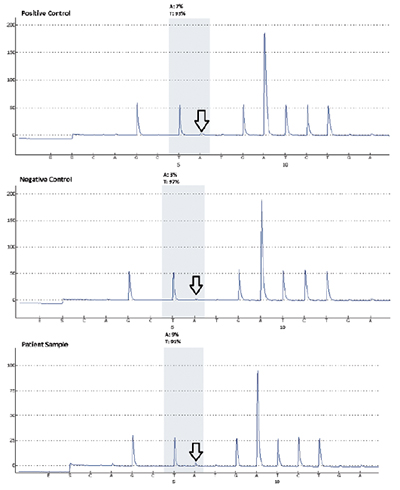
Fig. 3. Appropriate positive and negative controls are seen (as indicated by the arrows) for the BRAF V600E pyrosequencing assay followed by results of the patient sample which indicate the presence of a mutation. The test sensitivity or low limit of detection is validated at five percent mutant allele.
For this case, we first attempted to manually microdissect the tumor clusters from one of the other positive lymph nodes. LCM was performed as a salvaging means to recover DNA with higher tumor content after the pyrosequencing results showed an equivocal result. Applying LCM does have disadvantages. The equipment required for the process can be expensive and the process itself is time-consuming and labor-intensive. Also, when performing this technique, these tissue sections are not coverslipped and stained in gray and white colors, which results in a limited optical resolution that sometimes makes it difficult to identify tumor from normal tissue. This might be why LCM has largely been replaced by manual tissue microdissection that requires only designation of tumor areas by a pathologist followed by direct tissue scraping from the unstained slides. Nevertheless, the role of LCM in precise tissue microdissection in certain clinically challenging cases cannot be played by a manual-based approach.
Conclusion. In summary, this case shows the utility of certain microdissection techniques like LCM, especially in cases in which there may be only small microfoci of tumor cells present. In these situations, techniques that allow for precise dissection with minimized contamination from non-neoplastic cells are essential for quality molecular characterization of the tumor that may have potential therapeutic implications for the patient.
- Fend F, Raffeld M. Laser capture microdissection in pathology. J Clin Pathol. 2000;53(9):666–672.
- Hunt JL, Finkelstein SD. Microdissection techniques for molecular testing in surgical pathology. Arch Pathol Lab Med. 2004;128(12):1372–1378.
- Poynter JN, Elder JT, Fullen DR, et al. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma Res. 2006;16(4):267–273.
- Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29(15):2046–2051.
- Tang KT, Lee CH. BRAF mutation in papillary thyroid carcinoma: pathogenic role and clinical implications. J Chin Med Assoc. 2010;73(3):113–128.
- Jin M, Hampel H, Zhou X, et al. BRAF V600E mutation analysis simplifies the testing algorithm for Lynch syndrome. Am J Clin Pathol. 2013;140(2):177–183.
[hr]
Dr. Sharma is a pathology resident (PGY3) and Dr. Wang is an assistant professor in the Department of Pathology and Laboratory Medicine at the University of Florida College of Medicine, Jacksonville, where Dr. Wang is medical director of molecular pathology.
[hr]
Test yourself
Here are three questions taken from the case report. Answers are online now at www.amp.org/casereports and will be published next month in CAP TODAY.
1. Which of the following statements regarding laser capture microdissection is true?
a) The LCM technology was developed for research use only.
b) LCM is still used in certain clinical settings, especially when precision tissue microdissection is demanded for quality DNA samples with high wild type DNA proportion.
c) Manual tissue microdissection is cost-efficient and time-efficient and has replaced LCM clinically as the only means to isolate tumor DNA.
2. Manual tissue microdissection denotes:
a) Manually setting aside a piece of tumor tissue during specimen grossing for DNA preparation.
b) FFPE tissue blocks are manually punched followed by standard procedures of DNA isolation.
c) Tumor areas need to be designated and marked by a pathologist before the manual scraping of tissue from the unstained slides.
3. BRAF V600E mutation is indicated:
a) In metastatic malignant melanoma only as a companion test for targeted therapy.
b) In diagnosis of Lynch syndrome as a reflex test in patients with MSI-H genotype colorectal carcinoma.
c) As a companion test for non-small cell lung cancer as recommended (as standard practice) by the NCCN.