HBsAg tests
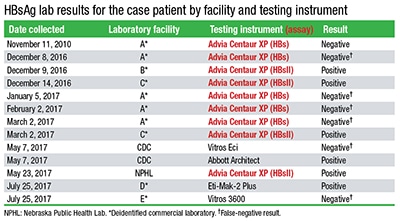
In “HBsAg tests, mutation in public health spotlight” (April 2018), you reported correctly that a patient had a false-negative hepatitis B surface antigen test performed on a Siemens Advia Centaur XP using the older HBs assay. However, the accompanying table (from MMWR Morb Mortal Wkly Rep. 2018;67:311–312) listed the Advia Centaur XPT. You published a note below the table to clarify that Lab A was in fact not using the Centaur XPT: “CAP TODAY was informed at press time that Lab A was not using the Advia Centaur XPT at the time of the false-negatives as it [the laboratory] first reported and as reflected in the table. It was using the Advia Centaur XP, which is compatible with the older HBs assay as well as the HBsII that was adapted to detect some of the common HBsAg mutations. HBsII was used at Labs B and C and at the NPHL.”
However, there is still confusion. We at Siemens Healthineers request that you go one step further and publish our revised table that reflects the true state of testing that led to the false-negative results on the Centaur XP using the older HBs assay. The root cause of the false-negatives was not related to the new HBsAgII assay as the MMWR table suggests in listing the Advia Centaur XPT. The Advia Centaur XP is compatible with both the HBsAg assay (HBs) and the HBsII assay. The XPT can run only the HBsII assay, which was developed to detect additional HBsAg mutations not detected by HBs, especially those arising in the ‘a’ determinant.
Siemens Healthineers routinely seeks opportunities to improve its products in order to achieve its goal of clinical excellence.
Melanie Pollan, PhD, MT(ASCP)
Director, Scientific and Clinical Affairs
North America, Laboratory Diagnostics
Vera Bitcon, MS, MT(ASCP)
Director, Infectious Diseases
Global Marketing, Laboratory Diagnostics
Siemens Healthineers
Tarrytown, NY
Electronic cancer checklists
I was excited to see the article “Clearing the air for electronic cancer checklists” (May 2018) and would like to share what we at Sunquest are doing to contribute to adoption of the electronic cancer checklists.
Sunquest is continually improving the PowerPath Synoptics tool set, which is included as part of our base system. We have introduced enhancements to our synoptic worksheets editing tool based on pathologists’ feedback to improve usability, streamline the completion of required fields, and deliver real-time previews of report formatting. This new version of the Synoptics tool includes a basic rules engine to suggest specific checklists and auto-populate answers based on specimen and case information. A rules-based approach can provide more flexibility for pathologists who find customizations necessary. The team has also upgraded our conversion tool enabling us to reduce turnaround time in delivering published CAP updates to PowerPath clients for implementation.
Our goal with these enhancements is to help accelerate the use of the eCCs, resulting in greater standardization in pathologist reporting and the creation of rich, minable, discrete diagnostic data. The searchable, discrete data of the synoptic module is valuable not only for cancer registry reporting but also for collecting and reporting required metrics for the Merit-based Incentive Payment System. To meet these reporting needs, Sunquest PowerPath offers a robust compound query tool, which allows customers to build complex searches based on patient, case, and checklist data. The value proposition for adoption of eCCs is significant and continues to grow.
One of the key goals of the cancer protocols is improved communication of all pertinent pathologic information to the clinician for proper staging and treatment. As a pathologists’ assistant, I believe there is an opportunity going forward to expand the focus on the synoptic format to ensure complete and required information is conveyed to the pathologists, as portions of the eCC data are derived from the gross description. The American Association of Pathologists’ Assistants recently introduced macroscopic examination guidelines for cancer resections to provide guidance to lab personnel performing gross examination and dissection. They are based on the CAP cancer protocols. Traditionally, gross information has been a lengthy narrative that varies in format and structure across pathologists’ assistants, making it challenging to parse specific data from dense paragraphs.
The Sunquest PowerPath Synoptics tool allows for custom templates to be built from scratch, and our clients use this functionality to create gross templates for cancer resections (similar to the CAP protocols with required data elements) to drive standardization and efficient extraction of data for staging by the pathologist. When the eCCs and gross synoptic templates are combined, the search and data mining possibilities are substantial. I foresee a full, streamlined synoptic reporting workflow spanning gross and microscopic information.
I look forward to Sunquest’s continued collaboration with the CAP and mTuitive, which provides our electronic cancer checklist solution for our CoPathPlus product, to advance cancer reporting.
Amanda Coble, MS, PA(ASCP)CM, MHA
Product Manager
Sunquest PowerPath
Sunquest Information Systems
Tucson, Ariz.