Charna Albert
April 2022—Don’t be afraid of livers.
Maryam K. Pezhouh, MD, offered that advice in a CAP21 presentation on autoimmune hepatitis, primary biliary cholangitis, and overlap syndrome, part of a session on common queries in liver pathology. “You don’t need to know everything when you’re looking at the liver,” said Dr. Pezhouh, associate clinical professor of pathology at the University of California, San Diego. “But you need to know what your clinician and your patient are asking.”
The main question: What is the major pattern of injury? Next is the degree of activity—is it mild, moderate, or severe? Sometimes a grade is assigned to the degree of activity, but Dr. Pezhouh prefers to be descriptive, “because a number is an absolute, and sometimes it won’t show the actual pathologies happening. For example, you have mild steatosis, but you have moderate inflammation around it. If I use different adjectives,” she explained, “I can tell them what I’m seeing.” The third question is the degree of fibrosis. Is it fibrosed yet, and if so, how much?
At the end of the report “I put everything together,” she said. “I say, I see this on the scope, and this is what you tell me the patient has, and I put two and two together and come up with a differential diagnosis.”
“Sometimes you are lucky,” in that what it is is clear, “but most of the time, especially in the liver, I’m not that lucky. I can narrow it down and tell them the pattern is hepatitic, or the pattern is in the bile ducts, or it’s cholestatic, and the differential diagnoses are different in each category.”
When Dr. Pezhouh receives a liver biopsy, she first examines the tissue at low power to look for the major pattern of injury, then at high power, after which she identifies the predominant morphologic pattern of injury. Next, she turns to the patient history to correlate her findings with the clinical information, and she calls the hepatologist to discuss the patient if necessary. “When I have all the clinical information, I go back to the slides to reevaluate and reach my final determination of the possible etiologies that can cause this pattern of injury.”
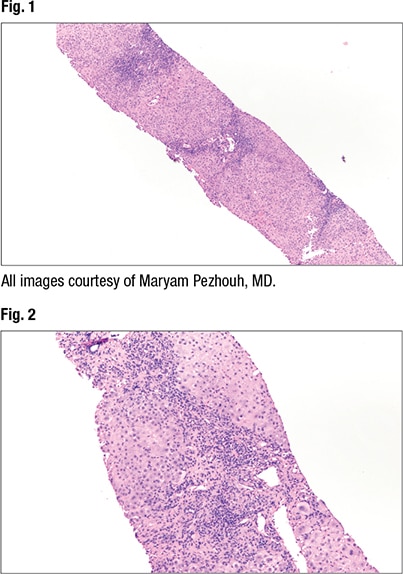 At low power (2× and 4×) the predominant histopathologic pattern of injury can be identified, Dr. Pezhouh said. “Is it fatty, is the inflammation portal, lobular, is it both? Is there necrosis, is there ischemia, is there sinusoidal dilation?” On low power, she said, “I think of different categories for diseases that can involve the liver.” And then at 10× or higher power she looks for specific clues, such as the type of fat (macro- or microvesicular, or evidence of steatohepatitis) and type of inflammatory cells. She also looks for other clues—Mallory bodies, apoptosis, single cell hepatocyte necrosis, and ischemia, for example. “Is there something that doesn’t belong there?”
At low power (2× and 4×) the predominant histopathologic pattern of injury can be identified, Dr. Pezhouh said. “Is it fatty, is the inflammation portal, lobular, is it both? Is there necrosis, is there ischemia, is there sinusoidal dilation?” On low power, she said, “I think of different categories for diseases that can involve the liver.” And then at 10× or higher power she looks for specific clues, such as the type of fat (macro- or microvesicular, or evidence of steatohepatitis) and type of inflammatory cells. She also looks for other clues—Mallory bodies, apoptosis, single cell hepatocyte necrosis, and ischemia, for example. “Is there something that doesn’t belong there?”
In her report, she describes the most prominent pathologic finding first. If the main finding is a fatty liver, for instance, “that should be your first line. And then you start describing everything else,” such as the portal tracts pathology, lobular pathology, and special stains performed, followed by a summary of the findings with the clinical and laboratory correlation. “Put everything together. Don’t just say, ‘This is chronic liver inflammation.’ Your hepatologist wants a differential diagnosis.”
For a specimen to be considered adequate, six to eight complete portal tracts are needed. To stage or grade chronic hepatitis, the specimen should be no less than 20 to 25 mm long, with at least 11 complete portal tracts. If the specimen isn’t adequate, Dr. Pezhouh reports that, since it can limit her ability to determine the cause of liver injury or amount of fibrosis.
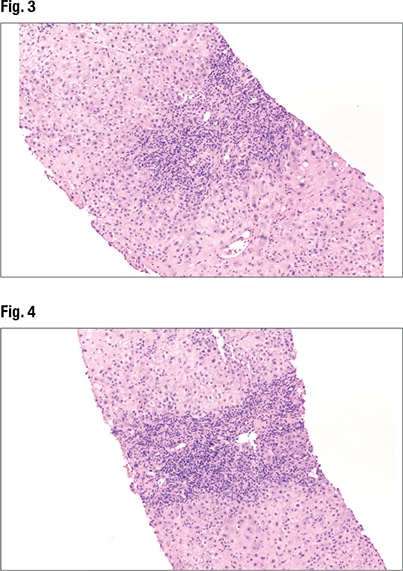 The most common patterns of liver injury, she said, are fatty liver disease, hepatitic (acute, chronic, or acute on chronic), chronic biliary/cholestatic, giant cell hepatitis, necrosis/ischemia, and vascular disease. “And you can have a combined pattern,” she noted.
The most common patterns of liver injury, she said, are fatty liver disease, hepatitic (acute, chronic, or acute on chronic), chronic biliary/cholestatic, giant cell hepatitis, necrosis/ischemia, and vascular disease. “And you can have a combined pattern,” she noted.
Fig. 1 is from a case Dr. Pezhouh shared. “It looks very blue,” with lymphocytes throughout and inflammation in the lobules and portals. On high power (Fig. 2), “a split out of inflammation in the lobules encasing the hepatocytes” can be seen. “At this point I know I’m looking at a hepatitic pattern of injury”—chronic inflammation in the lobules and portal tracts. The main differential in the hepatitic pattern is viral hepatitis, “or you’re dealing with autoimmune hepatitis, or a drug reaction,” she said.
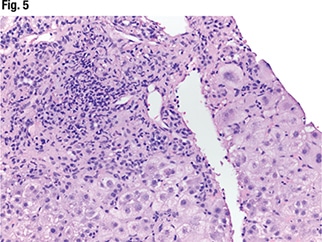
Under higher power (Fig. 3), interface hepatitis—in which inflammation enters the lobules—can be seen, as can inflammation in the portal tracts. In Fig. 4 (center), “there’s some spillage of the inflammation in the lobules but it’s mainly staying in the portal tracts at this point.” Under higher power (Fig. 5), “there are tons of plasma cells,” and lymphocytes are seen in the hepatocytes. “It’s somewhat rosetting the hepatocytes” (mid-left, bottom). “So there’s inflammation in the portal tracts, it’s spilling out tons of plasma cells, and there’s a selection of them going into the lobules and encasing the hepatocytes.”
The patient in this case is a 42-year-old female with a past medical history of ulcerative colitis who presented with dark urine and abdominal pain. With any case of ulcerative colitis, Dr. Pezhouh said, “you need to think about primary sclerosing cholangitis. But not every person who has ulcerative colitis has PSC.” The AST was highly elevated (967); ALT was 1524. Alkaline phosphatase was barely elevated (152), and bilirubin was normal (3.4). Antinuclear antibody was highly elevated (1:1280). Anti-smooth muscle antibody was negative. Ferritin was elevated (1780), and IgG was twice the normal level (2020). Ceruloplasmin was normal and the viral serologies negative.
“So at this point, based on everything, I favor autoimmune hepatitis,” Dr. Pezhouh said. “You have IgG elevation, you have positive autoimmune markers.” It’s more likely to be autoimmune than viral or a drug reaction at this point, she said, due to a constellation of findings.
 In cases of autoimmune hepatitis, AST and ALT are often more than twice the upper limit, as seen in this patient. GGT and alkaline phosphatase usually are less than twice the upper limit, “and the same was true in our case,” she said, with elevated AST/ALT the most predominant finding. Of the autoantibodies, “in our case only ANA was elevated, but most often”—in 50 percent of cases—“you have both ANA and anti-smooth muscle antibody elevation” (ANA elevation alone, 10 percent of cases; ASMA elevation alone, 30 percent). LKM and LC1 elevation (liver/kidney microsomal and liver cytosol antibody) occurs in some cases (five percent), “and it’s usually type two autoimmune.” IgG is often more than twice the upper limit.
In cases of autoimmune hepatitis, AST and ALT are often more than twice the upper limit, as seen in this patient. GGT and alkaline phosphatase usually are less than twice the upper limit, “and the same was true in our case,” she said, with elevated AST/ALT the most predominant finding. Of the autoantibodies, “in our case only ANA was elevated, but most often”—in 50 percent of cases—“you have both ANA and anti-smooth muscle antibody elevation” (ANA elevation alone, 10 percent of cases; ASMA elevation alone, 30 percent). LKM and LC1 elevation (liver/kidney microsomal and liver cytosol antibody) occurs in some cases (five percent), “and it’s usually type two autoimmune.” IgG is often more than twice the upper limit.
A range of patterns is seen in autoimmune hepatitis, including fulminant and acute and chronic hepatitis and cirrhosis. “In fulminant hepatitis you see lots of necrosis and ischemia,” Dr. Pezhouh noted. But the main histologic features of autoimmune hepatitis are plasma cell rich portal tract inflammation, interface activity, and lobular inflammation (regenerative rosettes, emperipolesis). And as always, “you have to have compatible laboratory and histologic findings,” and other causes must be excluded. “If I see only plasma cells,” she said, “I’m not going to call it autoimmune.”
In Fig. 6 is autoimmune hepatitis with fulminant liver failure and areas of necrosis (left side of liver core). In such cases she notes the percentage of necrosis. “And I correlate with the laboratory findings.”
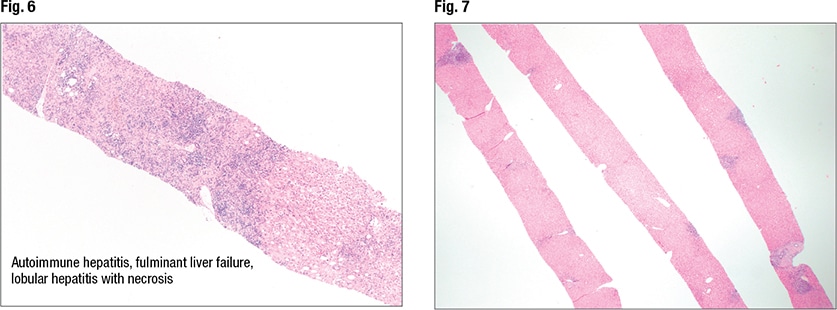
In Fig. 7 is an image from a second case. “This is an adequate biopsy,” Dr. Pezhouh said. “I have at least three cores of liver; they seem long, they don’t seem fragmented.” There is inflammation and it appears to be in the portal tracts. The lobular inflammation looks slightly dispersed, “but at this power it’s hard to see lobular activity.” At slightly higher power (Figs. 8 and 9), “again I see inflammation in the portal tracts but not much in the lobules. The portal tracts [right and left sides of liver core] are expanded by inflammation.” On higher power (Fig. 10) there are areas that show bile duct injury. Lymphocytes are rimming around and in between bile duct epithelium, causing bile duct injury, she said, indicating an irregular bile duct (mid-left). Another bile duct (Fig. 11, center) looks rounder, “but still you see lymphocytes in between the epithelium and it doesn’t look happy; it’s a little pinkish.” It seems that bile ductitis is present.
 This case is a middle-aged female patient who presented with fatigue and itching. AST/ALT were barely elevated (45 and 35, respectively). Alkaline phosphatase was highly elevated (680), and bilirubin was normal. ANA and anti-smooth muscle antibody were negative, and antimitochondrial antibody (AMA) was positive. IgG was normal, as was ceruloplasmin, and the viral serologies were negative. “You have antimitochondrial antibody positivity in a female presenting with fatigue and with elevation of alk phos and GGT. Thus, I’m probably dealing with primary biliary cholangitis” (formerly known as primary biliary cirrhosis). It’s more often seen in women, with the male to female ratio about 1:8. Alkaline phosphatase usually is more elevated than AST and ALT, and 95 percent of cases are AMA positive. But negative AMA isn’t a dealbreaker, she cautions. One should remember that around 10 percent of cases present with ANA positivity (often in the lower range) or positive anti-smooth muscle antibody.
This case is a middle-aged female patient who presented with fatigue and itching. AST/ALT were barely elevated (45 and 35, respectively). Alkaline phosphatase was highly elevated (680), and bilirubin was normal. ANA and anti-smooth muscle antibody were negative, and antimitochondrial antibody (AMA) was positive. IgG was normal, as was ceruloplasmin, and the viral serologies were negative. “You have antimitochondrial antibody positivity in a female presenting with fatigue and with elevation of alk phos and GGT. Thus, I’m probably dealing with primary biliary cholangitis” (formerly known as primary biliary cirrhosis). It’s more often seen in women, with the male to female ratio about 1:8. Alkaline phosphatase usually is more elevated than AST and ALT, and 95 percent of cases are AMA positive. But negative AMA isn’t a dealbreaker, she cautions. One should remember that around 10 percent of cases present with ANA positivity (often in the lower range) or positive anti-smooth muscle antibody.
Histologically, there will be patchy or often moderate portal tract inflammation, and there can be bile duct lymphocytosis and injury. “Look for both—not only lymphocytes in the bile duct, but you want to see the injury,” Dr. Pezhouh said. In about 40 percent of cases granulomas can be seen, either in the lobules or in the portal. “So it’s not a dealbreaker if you don’t see granulomas.” (Dr. Pezhouh provides a sample report after each case.)
Dr. Pezhouh presented a third case, one in which portal tract and lobular inflammation can be seen (Figs. 12 and 13), as can granulomas in the lobules (Fig. 14, center). “So basically I had a hepatitic pattern of injury and granulomas,” and granulomas have a long list of differentials: primary biliary cholangitis; sarcoidosis, common variable immunodeficiency, and other systemic granulomatous diseases; drug effect; infection; paraneoplastic syndrome. And sometimes they can be idiopathic, she said.
Bile duct injury is present in this case as well. “I have everything that we saw in PBC, and on top of that,” she said, there’s interface hepatitis (Fig. 15), caused by plasma cells. Emperipolesis is also present. So there are plasma cells, inflammation, and lymphocytes, “and it’s going inside and causing the interface hepatitis and inflammation in the lobules.”
This was a case of overlap syndrome, Dr. Pezhouh said. “And clinically it fits well.” The patient had elevated liver enzymes, alkaline phosphatase, and IgG, as well as compatible histology and pathology.
The most commonly seen overlap syndromes are autoimmune hepatitis and primary biliary cholangitis, though autoimmune hepatitis and primary sclerosing cholangitis overlap also can occur, most commonly in children and young adults. Primary biliary cholangitis and primary sclerosing cholangitis overlap is possible but “extremely rare,” she said, “so I try not to diagnose this unless it’s really screaming PSC/PBC.”
Overlap syndrome is not common (one to five percent of cases). Either autoimmune hepatitis or primary biliary cholangitis tends to dominate the clinical, serological, and histological findings, she said. In the example case “PBC was slightly dominating, but the clinical scenario and presence of lobular inflammation, plasma cells, and elevated AST/ALT and IgG” fit with autoimmune hepatitis. “So everything clinically was more autoimmune.” Sometimes overlap syndrome can manifest as a single primary with nonspecific overlap histologic findings, “so think about that as well.”
Two of three features of autoimmune hepatitis and of primary biliary cholangitis must be present to call it either autoimmune or PBC overlap syndrome, per the Paris criteria for AIH-PBC overlap. For AIH, these features are ALT ≥ 5 ULN, IgG ≥ 2 ULN or positive anti-smooth muscle antibody, and interface hepatitis on histology. For PBC, they are alkaline phosphatase ≥ 2 ULN or GGT ≥ 5 ULN, AMA positivity, and florid duct lesion on histology.

Primary biliary cholangitis and autoimmune hepatitis overlap is a challenging diagnosis, she said, because “AIH can show some spectrum of PBC. Sometimes you have expansion of the portal tracts with inflammation, and some of the inflammation goes to the bile duct and causes injury. So remember that AIH can show bile duct lymphocytosis or have patchy bile ductular proliferation.” If there is a mild degree of these, “back off a bit,” she advises. “It’s not necessarily both.”

Interface activity can be seen in primary biliary cholangitis, Dr. Pezhouh said, “but it’s usually minimal to mild.” And “you shouldn’t have widespread lobular inflammation—it should be minimal, focal, or somewhat mild.” Granulomas can be seen in autoimmune hepatitis, and “that’s the tricky point,” she said. But if they are present, “they will not be epithelial granulomas.” If the granulomas are widespread, consider PBC but look for other factors, such as AMA positivity and florid duct lesions.
“How do we approach a PBC or AIH overlap?” Look for clinical and serological findings that support a diagnosis of overlap, she said, per the Paris criteria. And the main histologic findings are as follows: More lobular hepatitis should be seen than expected in primary biliary cholangitis (which is minimal to mild) in a correct clinical scenario, and more bile duct injury than is typical of autoimmune hepatitis. “So when you have clinical AIH but you have bile duct injury and lymphocytosis, think of overlap. Or if you have AIH with weird things like cholestasis or ductopenia and/or cholate stasis—some sort of thing that shows biliary injury—think about overlap syndrome.”

In children and young adults, autoimmune hepatitis and primary sclerosing cholangitis overlap is often seen in the setting of ulcerative colitis or less commonly Crohn’s disease, Dr. Pezhouh said. It tends to present initially with a hepatitic pattern of injury, and then a component of PSC on follow-up. “Sometimes these patients don’t show much of a PSC portion at first but then eventually if you follow up they have some PSC component. So look for bile duct injury, bile ductular proliferation, and fibrosis around the bile ducts.” And look for alkaline phosphatase that’s more than twice the normal level. Finally, “always correlate with imaging of the extrahepatic biliary tree or ask them to do imaging if you suspect it,” she said.

Charna Albert is CAP TODAY associate contributing editor.