Karen Lusky
February 2022—Up first in a CAP21 molecular oncology tumor board session was an unusual case of head and neck cancer, one that raises questions about what salivary duct carcinoma is and the role of next-generation sequencing.
Justin Bishop, MD, the Jane B. and Edwin P. Jenevein, MD, distinguished chair in pathology and professor and chief of anatomic pathology, UT Southwestern, along with Saad Khan, MD, assistant professor of medicine (oncology) at Stanford, teamed up to present the case, along with a second case that will be reported in the March issue. (Dr. Khan practiced at one time at UT Southwestern, so this wasn’t their first tumor board together.)
Dr. Khan described the first case as one in which “pathologic diagnosis remains a real challenge, where using traditional stains is very difficult, and the clinical behavior is uncertain.”
The case is that of a 56-year-old woman who presented after three years of left parotid mass enlargement slowly growing over time. An FNA indicated epithelial cells in a stromal background suggestive of a salivary gland neoplasm of unclear etiology. CT imaging indicated an asymmetrically enlarged left parotid mass with enlarged cervical lymph nodes on the same side. The recommendation was surgical resection with removal of lymph nodes.
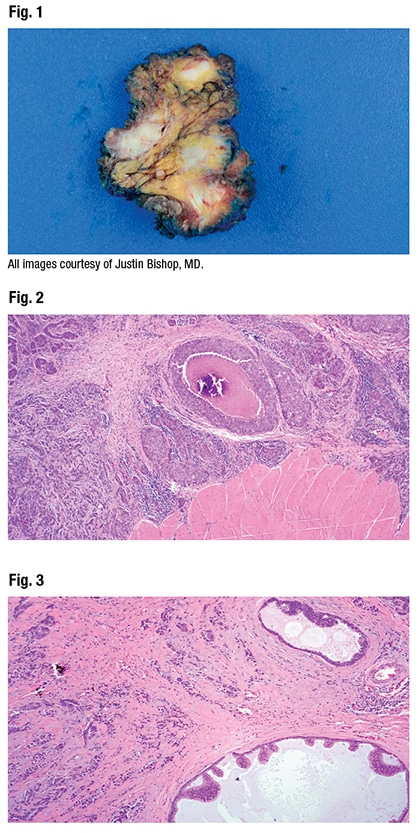
In the parotid gland (Fig. 1) were what Dr. Bishop said appear to be multifocal masses. “Some of these end up being lymph nodes,” but there are “very irregular tan white-appearing masses on gross examination.”
On histology, at low power (Fig. 2), they see it is a “bad tumor,” he said, “very infiltrative,” with skeletal muscle at bottom right. “This tumor is invading quite extensively beyond the parotid gland into nearby periparotid tissues as these kind of variably sized nests and cords of cells.” At top center he noted the comedo-type pattern necrosis.
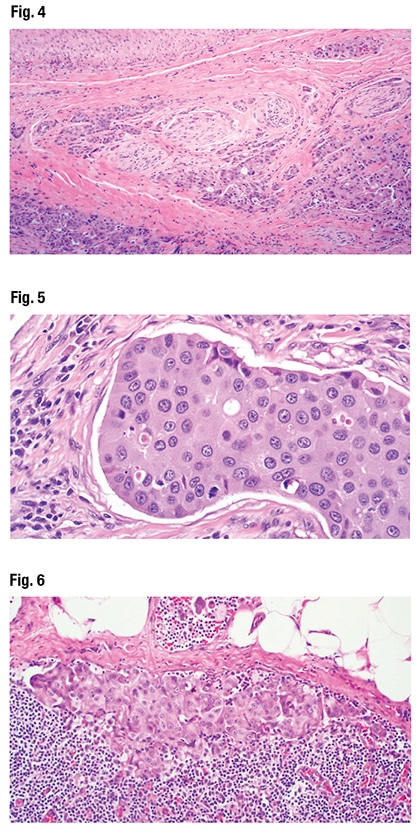
In Fig. 3 Dr. Bishop noted the infiltrative nests and cords of cells, and (at bottom and top right) cystic-like areas with “interesting architecture, this lollipop projecting into the cysts,” and a kind of “Roman bridging, and an apocrine-looking snout formation.” Perineural invasion was not difficult to find (Fig. 4). “So this is a very aggressive, high-grade tumor,” Dr. Bishop said.
At high power (Fig. 5), they notice the tumor cells have “abundant pink eosinophilic cytoplasm with a slight hint of granularity,” he said. There is some nuclear pleomorphism but the nucleoli are large. The chromatin is vesicular; many have a single prominent nucleolus. There’s “almost a vaguely squamoid look in areas, but no overt squamous differentiation at the histologic level.” Enlarged lymph nodes were seen on CT exam. “And some of the lymph nodes in the neck dissection were positive for metastatic tumor.” (Fig. 6).
 The differential diagnosis consists of salivary duct carcinoma, squamous cell carcinoma, high-grade mucoepidermoid carcinoma, metastasis, and high-grade adenocarcinoma not otherwise specified.
The differential diagnosis consists of salivary duct carcinoma, squamous cell carcinoma, high-grade mucoepidermoid carcinoma, metastasis, and high-grade adenocarcinoma not otherwise specified.
With p40 immunostaining (Fig. 7) the majority of the tumor is negative, he said, which rules out squamous cell carcinoma and mucoepidermoid carcinoma. In some of the cribriform areas (top left), “there appears to be a residual myoepithelial cell layer around it, suggesting this was either a precursor in situ lesion or, more likely, it’s extension of the tumor within a duct secondarily,” Dr. Bishop said.
BRST2 was strongly positive (Fig. 8). That the patient is a woman and BRST2 is a breast marker could suggest a metastasis from the breast, he said, but the estrogen receptor and progesterone receptor were negative. “And in fact the androgen receptor was incredibly strongly positive.” The BRST2 and androgen receptor, in addition to the morphology, indicated to Dr. Bishop that it was a high-grade carcinoma with apocrine differentiation.
It was sent out for next-generation sequencing to look for targetable alterations, “and this surprisingly had an ETV6-NTRK3 fusion, which was unexpected because this is the defining fusion for a mammary analogue secretory carcinoma.” Although not required, pan-TRK immunohistochemistry was done (Fig. 9). “And sure enough, this had diffuse nuclear staining.” (ETV6 rearrangement studies also could have been done, he noted, and they too would have been positive.) “So the final diagnosis in this case ends up being a high-grade salivary duct carcinoma harboring an ETV6-NTRK3 fusion. A very unusual case,” Dr. Bishop said.
Parotid masses can be static for a long time, Dr. Khan said, but then suddenly begin to change. “And there almost is a disconnect between the clinical course and the pathologic appearance, where it looks like it should be a very benign, almost non-malignant neoplasm. But when you look at the areas of necrosis, the invasion into the surrounding muscles and vasculature, you are shocked it’s been there for three years and didn’t double or triple in size during that time.”
 In this case, he said, the ETV6-NTRK3 fusion was not only critical in reaching the correct diagnosis but has significant treatment implications. Refining the diagnosis makes it possible, Dr. Khan said, “to parse out those salivary gland cancers that we think may be susceptible to broad-based anti-DNA destructive agents such as cisplatin and radiation, versus those that may be more precisely targeted genomically,” which increasingly in salivary gland cancers are able to be identified only with NGS.
In this case, he said, the ETV6-NTRK3 fusion was not only critical in reaching the correct diagnosis but has significant treatment implications. Refining the diagnosis makes it possible, Dr. Khan said, “to parse out those salivary gland cancers that we think may be susceptible to broad-based anti-DNA destructive agents such as cisplatin and radiation, versus those that may be more precisely targeted genomically,” which increasingly in salivary gland cancers are able to be identified only with NGS.
More and more salivary duct carcinomas are known to have fusions, Dr. Bishop agreed, and he said it has “humbled” him because he thinks of these fusions as being defining of lower-grade tumors. “ETV6-NTRK3 fusion means secretory carcinoma, for example, and yet we are starting to find these in salivary duct carcinomas. And it’s not only ETV6-NTRK3. We have found assorted RET fusions and even ALK fusions in some salivary duct carcinomas, all of which are targetable,” he said.
“It has changed my conceptualization of what salivary duct carcinoma is,” he continued. “I don’t think it is a distinct, single entity. I am becoming more aware that salivary duct carcinoma is a genetic wastebasket,” full of tumors that have the known PI3 kinase pathway mutations and that never have fusions, “but also a source of these de-differentiated fusion-driven tumors. And they look identical and stain identically.”
Dr. Bishop now routinely performs ALK and pan-TRK IHC and sends out for RET FISH testing. “And if I had the ability to send all of these for sequencing I would, because it is worth looking for these things. I’ve learned a lot from cases like this.”
When fusions are identified, Dr. Khan said, those who work in drug development know there’s a subset for which a drug must be found. “And many times those drugs are already available and we just need to bring those two together.”
 Dr. Khan likes Dr. Bishop’s use of the term “wastebasket” for salivary gland cancer, saying he made this very point to fellows with him in the clinic the day before. “I think pathologists understand this better than most oncologists do, which is when we say salivary gland cancer, you are really talking about a diverse group of very different diseases that are called salivary gland cancer just for convenience. And this applies more broadly toward head and neck cancer as well.”
Dr. Khan likes Dr. Bishop’s use of the term “wastebasket” for salivary gland cancer, saying he made this very point to fellows with him in the clinic the day before. “I think pathologists understand this better than most oncologists do, which is when we say salivary gland cancer, you are really talking about a diverse group of very different diseases that are called salivary gland cancer just for convenience. And this applies more broadly toward head and neck cancer as well.”
Nasopharyngeal cancer differs completely from HPV-positive oropharyngeal cancer, Dr. Khan noted, and from hypopharyngeal squamous cell cancer. “All of them carry the same diagnosis of squamous cell cancer and have treatment similarities, but they have completely different oncogenesis, completely different outcomes, and are very different in terms of how we expect their treatments to evolve in the future.”
Dr. Bishop’s view of looking for TRK has changed in recent years, he said. “I would say it’s worth looking for even if it’s a small number.”
What is your number threshold? Dr. Khan asked, likening such a number to that used in the medical oncology world to treat. “At what point do you think it becomes worthwhile to chase down something that might impact the care of one percent, 10 percent, 0.1 percent of people?”
“If I believe in my diagnosis,” Dr. Bishop replied, “then I’m not going to chase down TRK fusions in every salivary gland cancer, regardless of histology. I think that’s wasteful and very low yield.” He cites adenoid cystic carcinoma as an example. “I’m not aware of any cases with TRK fusions in that group. Same for acinic cell carcinoma, mucoepidermoid carcinoma, et cetera.”
“Would a single case report of adenoid cystic carcinoma with whatever fusion be enough?” he asks himself. “I don’t know. Probably not, but it’s a question I should consider.” When it comes to high-grade salivary duct carcinoma, he said, “there’s enough out there, published and unpublished, and it’s kind of gaining steam.”
NTRK tumors have a “tremendous deep response” to drugs like larotrectinib and entrectinib, Dr. Khan said, “and if you find it and the patient gets the drug, the response is amazing.”
“If you win that golden ticket and have the TRK fusion identified,” he said of the patient, “you will have a great time as far as cancer treatment is concerned. You will leave my office thinking I really know what I’m doing, when what I am doing is piggybacking off of the pathologist’s thorough knowledge, and all of what we know about TRK fusions, and this great drug that seems to work really well.”
Dr. Bishop said he recently saw a child diagnosed with an intermediate grade of mucoepidermoid carcinoma. “On re-review it was a secretory carcinoma, which completely changed their approach”—they considered the use of targeted therapy upfront instead of radiation given that the patient was a child.
How does Dr. Bishop decide to opt for a full NGS panel versus a focused panel? Dr. Khan asked.
“When it’s diagnostic,” Dr. Bishop replied, “the focused approach is ideal. Usually you have it down to between two things and this answer will take you one way. It’s rare that you are completely lost and have no idea.” For the case he presented, “a broader NGS approach would be ideal, and that’s what was done.” NGS identifies more and thus will generally be preferred, he noted. “It’s just what you have the ability to do.” Were every salivary gland cancer sent for NGS, he “would love it,” he said. “The data would be great not only for treatment but also for research.”
Karen Lusky is a writer in Brentwood, Tenn.