Karen Lusky
February 2018—A case of ALK-rearranged lung cancer was the subject of a multidisciplinary molecular tumor board presented last fall at CAP17 by pathologist Laura J. Tafe, MD, and oncologist Benjamin Levy, MD. Together they offered up insights into the tumor genomics of lung cancer with talk of testing guidelines, targeted therapies, resistance mechanisms, and circulating tumor DNA analysis.
Dr. Levy, assistant professor at Johns Hopkins School of Medicine, noted the expanding list of rarer yet actionable mutations in lung adenocarcinoma. “EGFR is the tip of the iceberg. It’s now ALK, it’s now BRAF, it’s ROS1,” he said in the session, “Molecular Oncology Tumor Board: Lung Cancer.”
The conversation an oncologist has with a lung cancer patient differs greatly depending on whether the patient has an actionable mutation. “We’re talking about giving patients oral targeted therapies where life expectancies are on the order of three to five years,” versus chemotherapy when the patient doesn’t have an actionable mutation and where life expectancies are much shorter, said Dr. Levy, clinical director of medical oncology and medical director of thoracic oncology, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center at Sibley Memorial Hospital, Washington, DC.
The focus of the session was a patient of Dr. Levy’s, a 36-year-old man diagnosed with metastatic lung adenocarcinoma. Dr. Levy suspected the man’s lung cancer might have an ALK rearrangement, given the patient’s age. For some time, however, the mutation escaped detection via tissue interrogation by FISH, for reasons later explained by Dr. Levy and Dr. Tafe, who is associate professor of pathology and laboratory medicine, Geisel School of Medicine at Dartmouth, and assistant director of the Laboratory for Clinical Genomics and Advanced Technologies, Dartmouth-Hitchcock Medical Center. They also highlighted some of the difficulties and new developments in diagnosing and treating ALK-rearranged lung cancer.
The 36-year-old man went to the emergency department to find out what might be causing his right arm to be swollen for nearly three days. The patient, a smoker, also complained of a nonproductive cough of several weeks’ duration. CT imaging identified a clot in his right internal jugular and a 2.6 × 2.8 × 2.8-cm cavitary lesion in the right lower lobe of his lung. He also had mediastinal lymph node involvement. An abdominal CT scan found a 3-cm lesion in the liver.
The patient underwent a staging and diagnostic CT-guided core biopsy of the liver lesion. The tissue demonstrated a poorly differentiated adenocarcinoma with signet ring features that was CK-7 and TTF1 positive. Sequencing with the Ion Torrent AmpliSeq 50-gene panel identified a TP53 mutation in the tumor but no actionable mutations. When the aforementioned testing was completed, no tissue remained to test for ALK or ROS1 rearrangements by IHC or FISH. “Managing tumor tissue for diagnostic and ancillary testing is a constant challenge in many cases,” said Dr. Tafe, who presented approaches laboratories are taking to optimize small tissues for molecular testing.
The patient went into a partial remission after four cycles of carboplatin and pemetrexed. During his first chemotherapy treatment, he had another liver biopsy, which provided enough tissue to test for ALK and ROS1 rearrangements by FISH. Both were negative. He continued to take maintenance pemetrexed chemotherapy.
ALK-rearranged lung cancer is a rare event in lung cancer, Dr. Levy said, noting that some community-based oncologists have asked him why they should test for ALK. They’ll never find it, they say, because in their patient population the prevalence is three percent. ALK does have a higher prevalence in certain patient populations, Dr. Levy said. Data show that in a never smoking patient population, for example, the prevalence of ALK is about 10 to 15 percent. “And then, interestingly, in one study, if you look at the patients who are never smokers and EGFR negative, the prevalence jumps up to about 25 percent. So you have to keep that in mind,” he said. ALK testing has to be done routinely in all lung adenocarcinoma patients, “but there is an enriched group of patients.”
Dr. Levy also noted that the Young Lung Cancer Study showed ALK had a higher prevalence than EGFR. The study is an observational one in which patients diagnosed with lung cancer under age 40 can register online and in which researchers mine their data.
Oncologist Barbara J. Gitlitz, MD, co-leader of the study, tells CAP TODAY that the latest update of the data was presented at the IASLC World Conference on Lung Cancer in December 2016. “And at that point, we had the results from the first 98 patients on trial, and 87 percent of them had adenocarcinoma. Of those who had stage IV adenocarcinoma [72 percent], 45 percent had an ALK rearrangement,” says Dr. Gitlitz, medical director of product development, oncology, at Genentech. Twenty-four percent of the stage IV adenocarcinoma patients were EGFR positive, seven percent were ROS1 positive, and 18 percent had other mutations, such as RET and ERBB2 (HER2). Very few of the patients were smokers. (Dr. Gitlitz and colleagues are finalizing the data and preparing a paper for submission.)
In the case at the center of the CAP17 session, the ALK rearrangement that was eventually discovered was due to a small intrachromosomal inversion “within chromosome 2,” Dr. Tafe said. “We see a rearrangement of the ALK gene with a partner gene and oftentimes this is EML4, but there are a number of other partner genes that have been identified and recognized. There are also multiple different breakpoints. The ALK usually breaks at exon 20, but the partner gene can have different breakpoints resulting in multiple transcripts.”
How should pathologists test for ALK? “Currently, there is an FDA-approved FISH assay and an IHC assay, which are practical to use,” she said. Because of the issue of multiple transcripts, reverse transcriptase PCR is not recommended because it is difficult to design an assay that is sufficiently comprehensive to detect all the isoforms, she said. Next-generation sequencing is increasingly becoming a viable alternative for ALK testing, though it usually has a longer turnaround time than a FISH or an IHC assay.
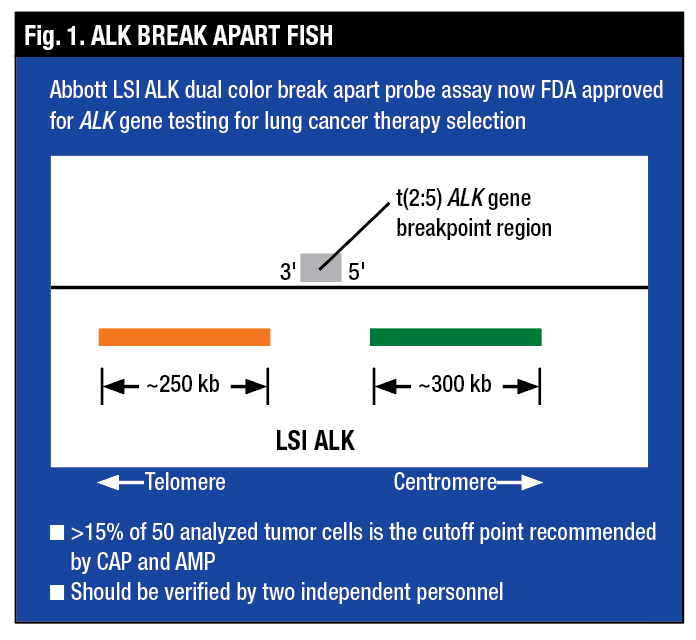
Dr. Tafe said the ALK Break Apart FISH assay is designed with an orange signal and a green signal flanking the ALK breakpoint. The FISH assay looks for ALK rearrangement only and is agnostic to the partner gene, “so it doesn’t matter what the rearrangement partner gene is; the test will tell you that the ALK gene itself is rearranged,” she said (Fig. 1).
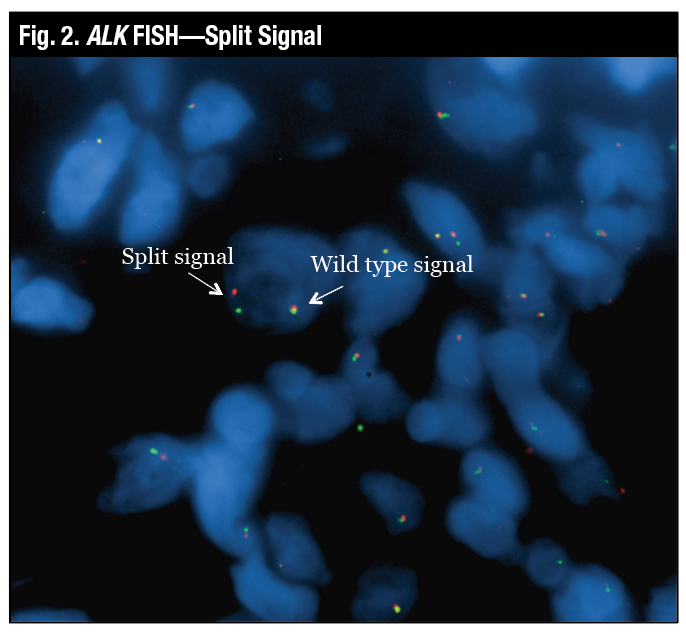
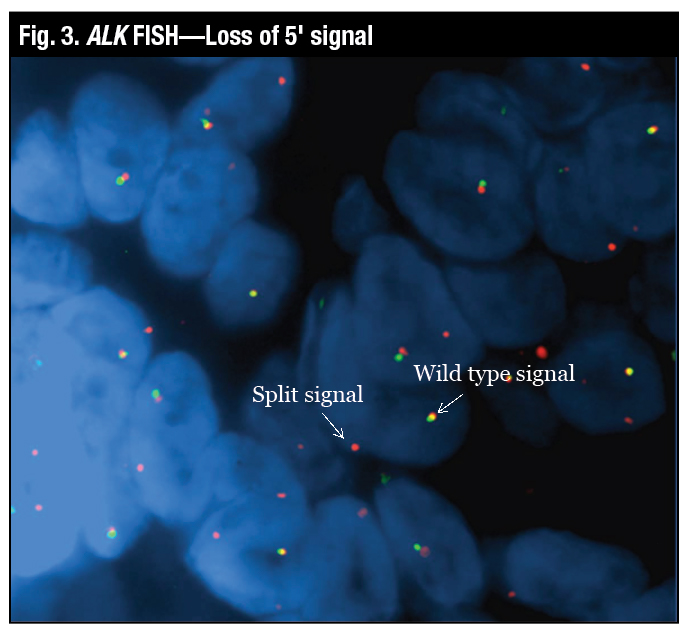
“The wild-type signal is the two probes close together, which looks yellow, and the true split signal is the separation of the spectrum orange and the spectrum green probes,” Dr. Tafe said (Fig. 2). Another variant of a positive signal that can be seen by FISH is the loss of the green or the 5′ signal, which is also deemed to be a positive rearrangement by FISH (Fig. 3).
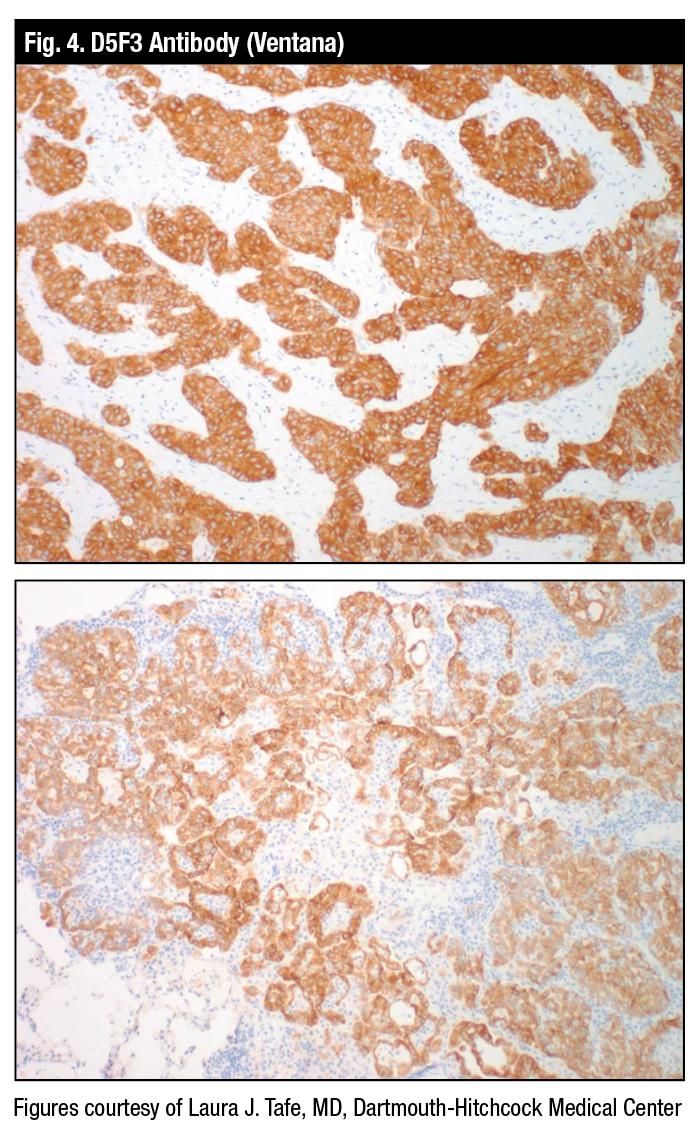
Dr. Tafe displayed an example of the FDA-approved Ventana D5F3 antibody that can be used frontline, she said, adding it need not be confirmed with FISH (Fig. 4). “It’s acceptable just to use the antibody.”
Dr. Tafe described the RNA-based anchored multiplex PCR sequencing assay, developed at Massachusetts General Hospital and available commercially as the Archer FusionPlex. “The beauty of this assay is that, similar to the ALK Break Apart FISH, you only need to know one gene you are targeting. So when targeting the ALK gene, for example, we have gene-specific primers and universal primers that allow the sequencing assay to identify what the rearrangement partner gene is.”
 The patient in the case presented in the session had eight cycles of maintenance pemetrexed chemotherapy, but the cancer advanced in his lung and liver. Dr. Levy offered him the option of a lung biopsy or a circulating tumor DNA plasma test. The patient chose the latter, which finally showed the ALK rearrangement by plasma, Dr. Levy said happily.
The patient in the case presented in the session had eight cycles of maintenance pemetrexed chemotherapy, but the cancer advanced in his lung and liver. Dr. Levy offered him the option of a lung biopsy or a circulating tumor DNA plasma test. The patient chose the latter, which finally showed the ALK rearrangement by plasma, Dr. Levy said happily.
“This is not circulating tumor cells. This is circulating tumor DNA that is shed from the tumor as a product of apoptosis and necrosis. We have more sensitive platforms that can now isolate the DNA from the blood and sequence it just like it would in tissue,” he said. “And you can get genetic interrogation off the blood and circumvent the need for tissue biopsies.”
Dr. Levy was not suggesting the tissue biopsy is not needed. “We do need tissue biopsies,” he said. “We need to make every attempt to get a biopsy up front.” He cited a University of Pennsylvania study that looked at concordance between the tissue and the circulating tumor DNA. “This is a trial where they mandated tissue biopsies,” and among about 100 patients in the study, there was sufficient tissue for molecular analysis for only 50. “If that’s happening at the University of Pennsylvania, I imagine that’s happening quite a bit in the community as well,” he said.
 The authors of the study wrote: “Actionable EGFR mutations were detected in 24 tissue and 19 ctDNA samples, yielding concordance of 79%, with a shorter time interval between tissue and blood collection associated with increased concordance (P = 0.038). ctDNA sequencing identified eight patients harboring a resistance mutation who developed progressive disease while on targeted therapy, and for whom tissue sequencing was not possible” (Thompson JC, et al. Clin Cancer Res. 2016;22[23]:5772–5782).
The authors of the study wrote: “Actionable EGFR mutations were detected in 24 tissue and 19 ctDNA samples, yielding concordance of 79%, with a shorter time interval between tissue and blood collection associated with increased concordance (P = 0.038). ctDNA sequencing identified eight patients harboring a resistance mutation who developed progressive disease while on targeted therapy, and for whom tissue sequencing was not possible” (Thompson JC, et al. Clin Cancer Res. 2016;22[23]:5772–5782).
In the case of the 36-year-old man, when the biopsy was performed twice and the ALK mutation could not be found, Dr. Levy reasoned that the patient was of an age at which ALK prevalence is high and he would therefore perform a plasma test. “It’s not that hard. We just do it [collect a blood sample] in the office and the turnaround time is seven to 10 days.” There aren’t enough data, though, on how patients who are plasma-positive and tissue-negative respond to ALK-directed therapies, he noted.
After receiving the positive circulating tumor DNA results, they retested the second biopsy using IHC and found it was positive for ALK. In work done in the past at another institution, Dr. Levy had seen a couple of negative FISH results on EML4–ALK that were positive by IHC on rebiopsy.
 Dr. Tafe called this “a really interesting point because this FISH assay is not like every other FISH assay.” The positive split signal can be very narrow on this assay, she said, “so you need very experienced readers to interpret this particular FISH assay.” What has been recognized is “there are discrepant cases that can be positive by FISH and negative by IHC or vice versa,” Dr. Tafe said (Marchetti A, et al. J Thorac Oncol. 2016;11[4]:487–495). “These cases are rare and patients may still respond to ALK inhibitor therapy.”
Dr. Tafe called this “a really interesting point because this FISH assay is not like every other FISH assay.” The positive split signal can be very narrow on this assay, she said, “so you need very experienced readers to interpret this particular FISH assay.” What has been recognized is “there are discrepant cases that can be positive by FISH and negative by IHC or vice versa,” Dr. Tafe said (Marchetti A, et al. J Thorac Oncol. 2016;11[4]:487–495). “These cases are rare and patients may still respond to ALK inhibitor therapy.”
Now diagnosed with ALK-positive lung adenocarcinoma, the patient began taking the ALK inhibitor alectinib (Genentech) in a clinical trial. Consequently, the cancer partially regressed, which was most obvious in the liver.
Dr. Levy pointed to “a tremendous groundswell of new therapies specifically for ALK,” with crizotinib the first ALK inhibitor for treatment-naïve patients. “I think the one that’s made the biggest splash for all of us has been alectinib,” he said, noting that the seminal work for alectinib was presented at the ASCO meeting in June 2017.
“Alectinib was compared head-to-head to crizotinib for ALK-rearranged lung cancer, and it doubled progression-free survival. We don’t have overall survival yet,” said Dr. Levy (Peters S, et al. N Engl J Med. 2017;377[9]:829–838). Patients remained on alectinib without tumor growth for two years on average. Dr. Levy suggested putting that in the context of what happens when a lung cancer patient without an actionable mutation is treated with chemotherapy: “Their tumor, on average, remains in check four to six months.”
 “The characteristics of alectinib, including its potent ALK inhibitory activity, high selectivity and activity against ALK secondary mutations resistant to crizotinib, and its CNS penetration, might contribute to the results seen in this study,” says Genentech’s Dr. Gitlitz. “Alectinib is active against brain metastases, which are seen in up to 60 percent of patients with ALK-rearranged lung cancer.”
“The characteristics of alectinib, including its potent ALK inhibitory activity, high selectivity and activity against ALK secondary mutations resistant to crizotinib, and its CNS penetration, might contribute to the results seen in this study,” says Genentech’s Dr. Gitlitz. “Alectinib is active against brain metastases, which are seen in up to 60 percent of patients with ALK-rearranged lung cancer.”
Ceritinib and brigatinib are also FDA approved for metastatic ALK-rearranged NSCLC. “Then we have lorlatinib just around the corner,” Dr. Levy said, predicting the FDA may approve the latter this year.
How long can metastatic ALK-positive lung cancer potentially be suppressed by sequencing the ALK inhibitors? “We don’t know yet,” Dr. Levy says. “We know alectinib is the best drug first. The drugs are coming out too quickly for us to understand optimal sequencing or even what the outcomes are with any sequencing right now. The general rule is these patients are living three to five years, but that doesn’t factor in newer therapies that are coming down the pike.”
The patient in the case had been on alectinib for just a year when his cancer began to progress in the lung and new bony lesions appeared. “We can do another liquid biopsy,” Dr. Levy told him, making clear to the patient that there is little understanding of the utility of plasma in identifying ALK resistance mechanisms. “We just don’t know. Or we can do another biopsy, and there are some resistant mutations that may help me select the next ALK-directed therapy,” he told him.
The patient chose the tissue biopsy, which showed the cancer had developed an ALK kinase domain G1202R resistance mutation and was PD-L1 positive (tumor proportion score >60 percent; 22C3 antibody). “Unlike EGFR-mutant lung cancer where EGFR T790M mutation is the most common resistance mutation,” Dr. Tafe said, “in ALK-rearranged lung cancer we are seeing a spectrum of resistance mutations in the ALK gene.”
It’s beginning to be learned that some of the ALK drugs may have specificity for certain mutations, Dr. Levy said. What’s emerging is G1202R, a secondary mutation that occurs mostly in patients post-alectinib. “And we have two drugs now that may specifically target G1202R, and that’s brigatinib and lorlatinib, but we just don’t know yet what to do.”
So what should be done for his patient?

Dr. Tafe
He has an ALK-rearranged lung cancer; he’s been treated with alectinib and now has disease progression. He has a rebiopsy that shows a potential actionable mutation, G1202R, but he also has PD-L1 positivity.” And the patient has seen advertisements about Keytruda and says, “‘I’m PD-L1 positive; you have to give me the immunotherapy.’ But what we know now,” Dr. Levy said, is patients with EGFR-mutant lung cancer and ALK-rearranged lung cancer don’t do well with immunotherapy. “In fact, they probably would do better with chemotherapy,” he said, citing a study titled “EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis” (Gainor JF, et al. Clin Cancer Res. 2016;22[18]:4585–4593).
In an interview with CAP TODAY, the study’s first author, Justin F. Gainor, MD, attending thoracic oncologist at Massachusetts General Hospital, said the study had two parts. In the first part, he and colleagues retrospectively looked at how 28 patients with advanced ALK-positive or EGFR-mutant lung cancer had responded to PD-1 and PD-L1 inhibitors. Twenty-two of the patients had EGFR mutations and six had ALK rearrangements.

Dr. Gitlitz
The response rate was “quite low,” says Dr. Gainor, noting it was under five percent. “None of the ALK-positive patients responded, but it should be noted that the total number of ALK-positive patients in this study was quite small. Further prospective assessments are needed to better define this.” The response rates in a separate cohort of 30 EGFR wild-type and ALK wild-type patients were around 20 percent. “And this is more consistent with what we have seen in clinical trials of these drugs,” Dr. Gainor says.
The second part of the study investigated why that may be the case. The researchers examined paired biopsy specimens from 62 EGFR-mutant and 19 ALK-positive lung cancer patients in another cohort. The biopsies were obtained from the patients before they had targeted therapy and when they developed resistance to the therapy. “And what we found is that a subset of EGFR– and ALK-positive patients do express PD-L1, but what was noteworthy is the [tumors] tended not to be inflamed, so they lacked CD8-positive tumor infiltrating lymphocytes [TILs]” before and after targeted therapy, says Dr. Gainor, who is also an assistant professor at Harvard Medical School.
The authors wrote, “Concurrent PD-L1 expression (≥5%) and high levels of CD8 + TILs (grade ≥2) were observed in only 1 pretreatment (2.1%) and 5 resistant (11.6%) EGFR-mutant specimens and was not observed in any ALK-positive, pre- or post-TKI specimens.”

Dr. Gainor
“PD-L1 expression,” Dr. Gainor says, “is commonly just a surrogate for inflammation since PD-L1 is upregulated in response to interferon gamma from infiltrating immune cells. In this study, we found that ALK-positive tumors generally lacked CD8-positive TILs even when they had PD-L1 expression.” This led the study’s authors to hypothesize that PD-L1 expression in EGFR– and ALK-positive patients may be due to innate immune resistance. They concluded in their article, “This lack of an inflammatory microenvironment, despite PD-L1 expression, is suggestive of innate immune resistance, and may limit the effectiveness of PD-1/PD-L1 inhibitors in these patient populations.”
Returning to the case study, Dr. Levy decided to prescribe another ALK inhibitor, brigatinib, for his patient in lieu of immunotherapy. The patient’s cancer partially responded. Later, however, the disease advanced quickly and the man died. “He lived about three and a half years” after diagnosis, Dr. Levy said, adding that if the patient hadn’t progressed so rapidly on the last ALK inhibitor, he might have tried immunotherapy next. “But it’s tough to know. We don’t know how to use these drugs in patients who are ALK positive.” Multiple trials are being conducted now in hopes of answering the question: Is there a role prospectively for immunotherapy for these rare genotypes?
“Unfortunately,” says Dr. Gainor, “we have seen that lung cancers treated with targeted therapies almost universally develop resistance, but what that resistance looks like can differ substantially based upon the ALK inhibitor that is being used.” Dr. Gainor says research he and his colleagues conducted showed that “the first-generation inhibitor, crizotinib, and the three second-generation inhibitors—ceritinib, alectinib, and brigatinib—each have different selectivity profiles and, as a result, the spectrum of resistance differs across the four” (Gainor JF, et al. Cancer Discov. 2016;6[10]:1118–1133). “The hope is to use repeat biopsies complemented by circulating [tumor] DNA to actually guide the selection and sequencing of ALK inhibitors rather than just empirically picking one followed by the other.”
As for using ctDNA up front, Dr. Levy says that, in retrospect, the patient in the case study should have had a liquid biopsy sooner but the technology was just coming out at the time. “So I think in hindsight, if there wasn’t enough tissue up front, I would have just done it up front. You have made a diagnosis with the biopsy, and there’s not enough tissue to complete the genetic testing; that is where I think circulating tumor DNA can help aid you in the diagnosis and also an understanding of the tumor biology.”
Dr. Tafe surmises that ctDNA testing may in time be performed up front to complement tissue testing or detect and monitor for resistance. “Right now, there are really no guidelines as to how to best validate these assays for clinical use and what indications for testing we should have” for their use. The updated CAP, International Association for the Study of Lung Cancer, and Association for Molecular Pathology guideline for selection of lung cancer patients for treatment with targeted TKIs, published online Jan. 22, says in some clinical settings in which tissue is limited and/or insufficient for molecular testing, physicians may use a cell-free plasma DNA assay to identify EGFR mutations (Lindeman NI, et al. Arch Pathol Lab Med. Epub ahead of print Jan. 22, 2018. doi:10.5858/arpa.2017-0388-CP). “But tissue helps us also make the diagnosis, confirm cancer, and classify what type of cancer it is, so it’s incredibly informative,” Dr. Tafe says.
Although the ctDNA assays have high positive predictive values, she said, “we don’t exactly know what the false-negative rate is for these assays in terms of the ALK-specific mutations. We know for EGFR, it’s probably around 15 percent or so, depending on the assay.” Various factors affect whether tumors release DNA into the circulation: tumor size, stage, and location and tumor vascularity, metastasis, and previous treatment. Thus, a major question, Dr. Tafe said, is how to deal with a negative ctDNA assay result when there is a high suspicion of either recurrence or metastatic disease. “And often the answer is to go back and get another tissue biopsy because there can be false-negative ctDNA cases.”
Dr. Levy said it’s difficult to get a false-positive circulating tumor DNA test result. “Anything that is considered a primary driver—EGFR, ALK, BRAF—and pops up is probably going to be true.” But the medical oncologist does need to communicate with the pathologist because if the pathologist finds something different in the tissue than was found in the blood, “then you have a problem.”
Then, too, he adds, open communication between the pathologist and medical oncologist is essential across the continuum of lung cancer care.
[hr]
Karen Lusky is a writer in Brentwood, Tenn.