CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the University of Washington, Seattle. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Shiva Khoobyari, MD
Eric Q. Konnick, MD, MS
 October 2018—Mosaic RASopathies result from postzygotic (“mosaic”) mutations in RAS-family genes.1 These disorders are characterized by dysregulation of RAS signaling pathways, resulting in overgrowth of affected tissues, and an increased risk of secondary transformation. Sensitive molecular diagnostic methods have been used to identify activating mutations in affected tissues and unravel the pathogenesis of the disorders.1-3 Nevus sebaceus and Schimmelpenning syndrome are mosaic RASopathies with an elevated risk of malignant transformation,1 and their accurate diagnosis has significant prognostic and therapeutic implications.4,5 Here, we discuss a clinical case in which genomic sequencing was applied to confirm the diagnosis of a potential Schimmelpenning/nevus sebaceus syndrome in a patient for whom there was a high clinical suspicion but an inconclusive microscopic assessment of grossly affected tissue.
October 2018—Mosaic RASopathies result from postzygotic (“mosaic”) mutations in RAS-family genes.1 These disorders are characterized by dysregulation of RAS signaling pathways, resulting in overgrowth of affected tissues, and an increased risk of secondary transformation. Sensitive molecular diagnostic methods have been used to identify activating mutations in affected tissues and unravel the pathogenesis of the disorders.1-3 Nevus sebaceus and Schimmelpenning syndrome are mosaic RASopathies with an elevated risk of malignant transformation,1 and their accurate diagnosis has significant prognostic and therapeutic implications.4,5 Here, we discuss a clinical case in which genomic sequencing was applied to confirm the diagnosis of a potential Schimmelpenning/nevus sebaceus syndrome in a patient for whom there was a high clinical suspicion but an inconclusive microscopic assessment of grossly affected tissue.
Case. A five-year-old African-American boy presented with a ~3.5 cm × ~2.0 cm tan papillomatous plaque on the left parietal scalp. His history was significant for cognitive developmental delay and epileptic seizures, with Fragile X syndrome testing showing one unexpanded FMR1 allele. Histologic examination of a 4-mm punch biopsy showed only a few primitive hair follicles and small sebaceous lobules (Fig. 1). Although the microscopic findings were nonspecific, the histologic differential diagnosis included skin with no diagnostic alteration, prepubertal stage of a nevus sebaceus, or other somatic overgrowth syndromes. Targeted next-generation sequencing using a clinically validated method6 (see “Methods”) was performed on 2.8 µg DNA extracted from a formalin-fixed, paraffin-embedded skin sample. Average sequencing coverage was 865-fold, and only an activating HRAS p.Gly13Arg mutation (NM_005343.2:c.37G>C) was identified. This mutation was detected in only 20 percent of sequencing reads (27 of 134 reads), supporting its presence in only a subset of the tested cells, consistent with somatic mosaicism. No mutations were detected in KRAS, NRAS, PTEN, or PIK3CA, which have also been described in similar lesions.
Discussion. Nevus sebaceus (NS) is a common congenital skin hamartoma, with the clinical appearance characterized by yellow, waxy, hairless plaques on the scalp, face, or neck.1 NS shows progressive growth and becomes thickened and verrucous as the patient matures.7 NS is typically a solitary lesion with no other clinical association. However, in approximately seven percent of cases, it is the hallmark lesion of Schimmelpenning syndrome (MIM: 163200),8 a multiorgan disorder characterized by the association of nevus sebaceus with extra-cutaneous abnormalities, including ocular, skeletal, cardiovascular, and central nervous system anomalies, including seizures. Sebaceous nevi develop along Blaschko’s lines, characterized by S- or V-shaped strips of affected skin.9 This clinical presentation suggests that the underlying etiology may be due to genetic mosaicism.10
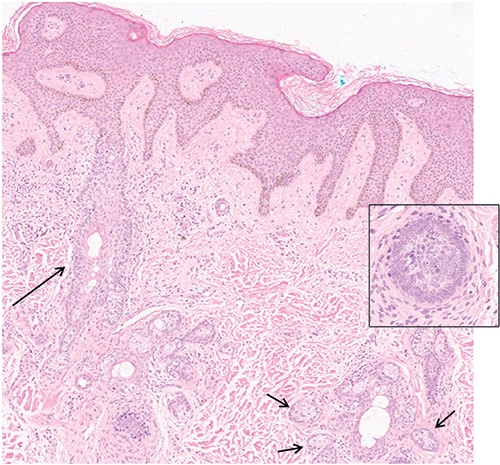
Fig. 1. Photomicrograph of the lesion shows dilated follicular infundibula (long arrow) with few attached budding undifferentiated hair follicles. There are small sebaceous glands (short arrows) next to dilated sebaceous ducts (hematoxylin-eosin stain; × 200). lnset: undifferentiated hair follicles (hematoxylin-eosin stain; × 400).
The phenotype of mosaic disorders is determined by when the mutation arises in development, and the clinical presentations are accordingly varied. In NS, it has been proposed that the genetic mosaicism is limited to the skin, while the mutation is observed in other organs in Schimmelpenning syndrome.10 Other conditions, such as hamartoma tumor syndrome and PIK3CA-related overgrowth syndrome, involve postzygotic mutations in PTEN and PIK3CA, respectively. These syndromes also can present with skin lesions in a patchy distribution, which raise suspicion for genetic mosaicism.11
The definitive treatment of nevus sebaceus is full-thickness dermal and epidermal excision; however, controversy exists on whether prophylactic excision is warranted.12,13 In addition to surgery, laser and photodynamic therapy have also been explored for treatment of nevus sebaceus, with varying degrees of success.13
Although secondary transformation of affected tissue is typically seen post puberty, it is not uncommon for malignancy to arise within nevus sebaceus during childhood.7 Secondary neoplasms occur in about 24 percent of sebaceous nevi and are primarily benign, most commonly trichoblastomas and syringocystadenoma papilliferum. However, malignant neoplasms such as basal cell carcinoma and squamous cell carcinoma have also been reported,13 with basal cell carcinoma being the most common malignant neoplasm arising in sebaceous nevi.14 These observations support ongoing, lifelong clinical assessment of lesions.
Molecular studies have provided opportunities to better understand the underlying genetic causes of nevus sebaceus and Schimmelpenning syndrome. Studies using Sanger and whole exome sequencing have revealed postzygotic activating mutations are frequent, with HRAS c.37G>C being the most common, followed by KRAS c.35G>A.1-3
While most cases of sebaceous nevi can be diagnosed on histologic examination, occasional cases, such as presented here, demonstrate that molecular techniques may be helpful for confirmation of diagnosis and may spare patients additional procedures. Possible scenarios for molecular testing include when microscopic assessment is inconclusive in the setting of reasonable clinical suspicion, or when skin biopsy cannot be performed in the setting of suspected Schimmelpenning syndrome. Possible substrates for molecular testing can include biopsy material, peripheral blood, or even lesional tissue obtained from scratched-off scurf.5 In such clinical scenarios, appropriate genetic testing can help prevent multiple biopsies or invasive procedures.
Methods. DNA sequencing was performed using the UW-OncoPlex assay on a HiSeq 2500 sequencing system (Illumina, San Diego) with 2 × 101 bp paired-end reads as previously described.6 Sequencing libraries were prepared from DNA obtained from FFPE samples. Libraries were hybridized to a custom design of complementary RNA (cRNA) biotinylated oligonucleotides targeting approximately 1.5 megabases of DNA from 262 genes involved in neoplasia. Sequence alignment and variant calling were performed against the reference human genome (University of California, Santa Cruz, hg19); alignment processing was performed using Burrows-Wheeler Aligner; and nucleotide variants were detected using Genome Analysis Toolkit (Broad Institute, Cambridge, Mass.), VarScan 2, and custom software. The UW-OncoPlex assay is capable of detecting all classes of mutations, including single-nucleotide variants, indels (1–100+ bp), and structural rearrangements, with a sensitivity of 10 percent minor allele frequency.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44(7):783–787.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133(3):827–830.
- Sun BK, Saggini A, Sarin KY, et al. Mosaic activating RAS mutations in nevus sebaceus and nevus sebaceus syndrome. J Invest Dermatol. 2013;133(3):824–827.
- Hucthagowder V, Cottrell CE, Schaffer A. Targeted next-generation sequencing identifies HRAS mutation in Schimmelpenning-Feuerstein-Mims syndrome. Poster presented at: Annual Clinical Genetics Meeting; March 8–12, 2016; Tampa, Fla.
- Wang H, Qian Y, Wu B, Zhang P, Zhou W. KRAS G12D mosaic mutation in a Chinese linear nevus sebaceous syndrome infant. BMC Med Genet. 2015;16:101.
- Pritchard CC, Salipante SJ, Koehler K, et al. Validation and implementation of targeted capture and sequencing for the detection of actionable mutation, copy number variation, and gene rearrangement in clinical cancer specimens. J Mol Diagn. 2014;16(1):56–67.
- Goldstein GD, Whitaker DC, Argenyi ZB, Bardach J. Basal cell carcinoma arising in a sebaceous nevus during childhood. J Am Acad Dermatol. 1988;18(2 Pt 2):429–430.
- Aslam A, Salam A, Griffiths CE, McGrath JA. Naevus sebaceus: a mosaic RASopathy. Clin Exp Dermatol. 2014;39(1):1–6.
- Jackson R. The lines of Blaschko: a review and reconsideration: observations of the cause of certain unusual linear conditions of the skin. Br J Dermatol. 1976;95(4):349–360.
- Happle R. Mosaicism in human skin: understanding the patterns and mechanisms. Arch Dermatol. 1993;129(11):1460–1470.
- Nathan N, Keppler-Noreuil KM, Biesecker LG, Moss J, Darling TN. Mosaic disorders of the PI3K/PTEN/AKT/TSC/mTORC1 signaling pathway. Dermatol Clin. 2017;35(1):51–60.
- Rosen H, Schmidt B, Lam HP, Meara JG, Labow BI. Management of nevus sebaceous and the risk of basal cell carcinoma: an 18-year review. Pediatr Dermatol. 2009;26(6):676–681.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29(1):15–23.
14. Fergin PE, Chu AC, MacDonald DM. Basal cell carcinoma complicating naevus sebaceus. Clin Exp Dermatol. 1981;6(1):111–115.
Test yourself
Here are three questions taken from the case report. Answers are online now at www.amp.org/casereports and will be published next month in CAP TODAY.
1. Which of the following is the most commonly mutated gene in Schimmelpenning syndrome?
a) KRAS
b) HRAS
c) NRAS
d) PTCH
2. Which of the following is the most common malignant neoplasm arising within nevus sebaceus?
a) Squamous cell carcinoma
b) Trichoblastoma
c) Basal cell carcinoma
d) Keratoacanthoma
3. Which of the following sequencing methods have been used to identify the underlying nevus sebaceus mutation?
a) Sanger sequencing
b) Whole exome sequencing
c) Targeted next-generation sequencing
d) All of the above
Dr. Khoobyari is a resident in the departments of Pathology and Laboratory Medicine, and Dr. Konnick is an assistant professor in the Department of Laboratory Medicine and associate director of the genetics and solid tumor laboratory, all at the University of Washington, Seattle.