Anne Paxton
March 2015—When the 11th Circuit U.S. Court of Appeals issued an opinion harshly criticizing the CAP’s guidelines on review of Pap tests last summer, many in the pathology community were stunned by the rebuke and wondered whether change was called for.
The CAP and the American Society of Cytopathology developed the guidelines in 1998 to ensure unbiased evaluation when cytopathologist and cytotechnologist Pap test findings are being litigated. The guidelines’ key recommendation is that pathologists conduct a blinded review, without knowing the result in advance, before testifying about a Pap test result in a malpractice case. Ruling in the case Adams v. Laboratory Corp. of America on July 29, 2014 (Laboratory Corp. of America, 760 F.3d 1322 [11th Cir.2014]), the Court of Appeals said the guidelines wrongly intruded into legal questions on expert witness testimony that should be left to the courts.
So far, College officials have decided to retain the guidelines. After reviewing the case, the CAP Cytopathology Committee, part of the Council on Scientific Affairs, decided in December that the “CAP Guidelines for Review of Pap Tests in the Context of Litigation or Potential Litigation” are more or less fine the way they are. The committee, which has conducted several other reviews of the guidelines over the years (in 2001, 2005, and 2012), has now recommended only minor edits.
The case began when Christina Adams and her husband filed suit against LabCorp, alleging that the company failed to properly identify abnormal cells on five Pap tests she had between 2006 and 2008; in 2009, Adams was diagnosed with cervical cancer that had already metastasized to her lymph nodes.
The Adamses retained the distinguished cytopathologist Dorothy Rosenthal, MD, as an expert witness to testify as to whether LabCorp’s employees had breached the standard of care for cytotechnologists in reviewing the slides. Dr. Rosenthal reviewed the slides at LabCorp’s laboratory in Atlanta, spending about 90 minutes examining them, and concluded that the LabCorp cytotechnologists’ review fell short of the applicable standard of care because it failed to identify abnormal cells that should have been identified.
LabCorp moved to exclude Dr. Rosenthal’s testimony, however, citing the CAP/ASC guidelines as evidence that a blinded review was the standard set by the profession. The U.S. District Court for the Northern District of Georgia agreed to exclude the testimony and granted LabCorp’s motion for summary judgment. The court expressed concern about the risk of bias in Dr. Rosenthal’s assessment and the general risk of review bias in non-blinded review. In fact, Dr. Rosenthal herself referred to “review bias” in her deposition, explaining that “any time you go to look at another case somebody else has looked at and rendered a diagnosis, you’re biased by what they called it, and you’re also biased if you have any additional information.”
The 11th Circuit Court of Appeals reversed the district court. The guidelines, the court said, “are not objective, scientific findings; they are not guidelines followed by laboratories to screen for pre-cancerous or cancerous cells; they are policy proposals to limit how the courts can find the members of the organizations liable for professional negligence when they are sued.” In its experience, the court added, the CAP guidelines are “the first time” that an industry group has attempted to define and limit the evidence courts should accept when the group’s members are sued.
Jack Bierig, who was general counsel for the CAP from 1980 to 2005 and helped develop the guidelines, disagrees with the 11th Circuit Court’s characterization of the guidelines. “The guidelines address the situation of a retrospective reviewer who knows that a plaintiff had cancer. Since the reviewer already has that knowledge when reviewing the slides, that knowledge has to affect his or her decision. By contrast, the original cytotechnologist or pathologist who reviewed them didn’t have that knowledge. The guidelines were designed to recommend a methodology designed to reduce the bias of hindsight. I personally don’t see anything wrong with that,” says Bierig, who is a partner with Sidley Austin LLP in Chicago.
Pathology had reason to be especially concerned about such bias because of a side effect of the passage of CLIA. In 1992, after CLIA ‘88 regulations were issued, “litigation really went up dramatically,” says Marshall Austin, MD, PhD, former member of and advisor to the CAP Cytopathology Committee and professor of pathology and director of cytopathology at the University of Pittsburgh Medical Center.
With the public attention and media hype about the pathologists and cytotechnologists behind the slides being linked to potential errors in diagnosis, “suddenly attorneys and patients were aware they could bring charges against someone for malpractice for missing cases,” says Barbara Crothers, DO, current chair of the Cytopathology Committee.
Cytology is different from any other screening test, Dr. Austin emphasizes. “Cytology slides have thousands of cells and in the screening process, where cytologists look at many, many cells per second over about five minutes, they typically don’t look individually at every cell, and they have to make a lot of interpretations based on variable morphology of the cells.” The closest analogy to reading a Pap test is viewing a mammogram, because it’s also based on visual interpretation, he says. Radiologists face some of the same issues because often, when it’s known that a patient has cancer, “they go back to the scan and say there must have been something not interpreted properly.”
While the history of CLIA is long and complicated, Dr. Austin says, Pap smears played a central role in the push for federal regulation of clinical laboratories. “CLIA was largely prompted because of concerns about what was going on in Pap smear labs. A few commercial labs figured out if you gave away Pap smears for almost nothing, akin to a ‘loss leader,’ and let the doctors’ offices bill the insurance company, the offices could get a markup and the labs retained other work.”

Dr. Crothers
The problem was that those laboratories were driving the cytotechnologists to look at way too many slides, he says. “Since the Pap smear is not a perfect test, it wasn’t hard to go back and find errors that could have potentially been avoided if the cytotechnologists were looking at fewer slides.” Since that time, there have been tremendous advances with cytology testing, he hastens to add. “But it’s still not a perfect process; nothing is perfect when it involves visual evaluation.”
Laboratories themselves may have been the unintended cause of the surge in malpractice suits, says Dr. Crothers, cytopathology medical director for Walter Reed National Military Medical Center, Bethesda, Md. “CLIA actually stated that for good laboratory practice, laboratories should rescreen pathology past cases where the patient has a current diagnosis of high-grade epithelial lesion, and look retrospectively at negative cases from the last five years to see if there were cases where they might have missed some cells.”
That was intended as a quality assurance measure, she says. “It was to help laboratories better develop their diagnostic eye and start to learn to recognize patterns that might be subtle and that are frequently missed. Generally that’s what a good laboratory would do, say, in a five-year review.”
Often such reviews find a high percentage of cases that are atypical in some way. “It can be as high as 50 to 90 percent in some cases,” Dr. Crothers says. “But that’s really just a reflection of the process, because sometimes these lesions are high in the cervical canal. They may not shed many cells or the cells may be degenerated, or the potentially atypical cells may be overlapped with other normal cells that occur on the Pap. Very tiny high-grade cells can sometimes be mistaken for endometrial cells. Or there are other factors such as inflammation, blood, or other obscuring cells that make the slide difficult, if not impossible, to interpret.”
“But once you know the patient has developed something, the review process is very intensive, and you scrutinize the entire slide trying to find something you can learn from.” When laboratories were essentially required by law to review and report, they started to issue amendments to pathology reports, reclassifying some Pap tests called negative in the past as “atypical squamous cells.” Public attention followed—and extreme reactions in some cases.
In Rhode Island, for example, a hospital was told by the state health department to rescreen a whole series of its patient slides and issue an amended report in any case where a later opinion said a slide was abnormal. “As a result of that, patients filed 15 lawsuits—even patients without cervical cancer. That was the kind of environment we were in at the time,” Dr. Crothers says.
In 1996, the CAP convened a conference devoted to liability and quality issues in vaginal and cervical cytology. “It was from that breeding ground that there came this upswell for some kind of guidance for labs and patients and plaintiff and defendants to have a realistic idea of what constitutes a failure of standard of care,” Dr. Crothers says. “It was really to recognize that the Pap test is subjective, that there are factors making it extremely difficult to interpret. And zero error tolerance is really not possible.”
“Some failures are true diagnostic errors where every cytopathologist or cytotechnologist looking at that slide would say ‘that’s definitely there and should have been caught.’” But the other type of error is an error in the realm of subjective interpretation, she says. “Those are the ones that cause the most lawsuits.”
While there may not have been a causal relationship, after the guidelines were issued there was a drop in litigation, Dr. Austin says. Pathologist David B. Troxel, MD, reported in 2012 that The Doctors Company, the largest insurer of physician and surgeon liability in the U.S., had an average of 19.7 gynecologic cytology claims per year from 1995 to 1997. Then from 1998 to 2003 the number declined to seven per year, subsequently falling to 1.4 per year from 2004 to 2010.
According to the 2012 article written by Dr. Troxel, there had been a significant decline in the number of claims involving melanoma, breast, and gynecologic cytology. Pathology typically has a low frequency of claims or demands for payment, but claim severity trends high. From 1999 through 2010, the average severity for all closed pathology claims was $431,964, for melanoma it was $753,100, and for gynecologic cytology it was $596,455 (Troxel DB. Am J Surg Pathol. 2012;36[1]:e1–e5).

Dr. Austin
Nevertheless, the data show gynecologic cytology is still in a small group that accounts for the majority of claims, and Pap tests are one of the areas likely to result in litigation, Dr. Austin notes. “So it’s gone down, but it’s still in that high-risk category.”
Telling a jury the truth about Pap tests is not always simple, Dr. Austin notes. “Although I’m primarily a defense expert, I’ve done some cases for plaintiffs, but I’ve always insisted that I would have to show that slides would be repeatedly misread in a blinded review type of exercise. We’ve done that as a first step in cases I’ve testified in, but of course other experts have avoided that because it takes some additional time and effort.”
The appeals court said that Dr. Rosenthal—whose testimony was excluded by the Georgia trial court—could testify without a blinded review, Dr. Austin points out. “It doesn’t say that blinded review is not a good method, just that experts like Dr. Rosenthal may testify without it and it’s up to the jury to sort it out.”
The standard of practice requires professional judgment, Dr. Austin points out. “And if professional societies can’t provide insight into that, then I don’t know who is supposed to do it. I think it’s intuitive that professional organizations in charge of training and accreditation programs will have special knowledge of standards of practice, and people who don’t understand that are very misguided.”
Bierig, who represents the American Medical Association, the American Board of Medical Specialties, and several certifying boards, agrees that the CAP and the ASC, in issuing the guidelines, acted completely within their proper scope. “All specialty societies that I know of have put out practice guidelines for how physicians in that specialty should diagnose, treat, or report various conditions. What’s different about the College’s guidelines is that they address how expert witnesses in a certain kind of case, cytopathology malpractice cases, should review slides they’re called upon to review. But there is nothing wrong with that. The College is simply expressing its opinion on an important issue on which its members have expertise.”

Bierig
The court certainly does not have to accept the College’s viewpoint, Bierig adds. “The court is not bound by what the College or ASC says. A court that disagrees with the College’s proposed approach is free to reject it. What is most troubling in this case is how the court attributes bad motives, malevolence, or bad faith to the College, and says that these guidelines were really biased and clearly designed to protect members of the College.” A medical society, just like a natural person, has a First Amendment right to express its views, he says.
The CAP guidelines are no different from an amicus brief, Bierig says. “The way the process works is that an entity sets forth its position to a court either in the form of an amicus brief or in some other way, such as a practice guideline. The court either agrees with that position or it doesn’t. But the notion that the entity is condemned for expressing its good faith position where the court disagrees with that position flies in the face of this country’s commitment to freedom of expression.”
Whether or not a court agrees with the position taken by the College on expert testimony in cytopathology cases, there can be no doubt that the guidelines were adopted in good faith, Bierig says.
“Testimony that doesn’t meet basic fairness guidelines can skew the adjudicative process. You can have a very sympathetic plaintiff, a woman who died or is dying of cervical cancer because her positive Pap test was missed, and the judge and jury can’t help but feel very sorry for that person,” Bierig says. “But the question is, was the pathologist or cytotechnologist negligent? Dr. Austin would say we want to have a fair evaluation of that question. To have a reviewer come in knowing that the slides were of a person who had cervical cancer, and say, ‘well, this should have been spotted’—that’s not the position the cytotechnologist or pathologist was in at the time of the review.”
“If you talk to cytopathologists, they will tell you that a cytotechnologist or a cytopathologist who is doing a very good job will still, from time to time, miss calling cases,” Bierig adds. “That doesn’t necessarily mean they are negligent, but that there is a certain irreducible number of false-negatives that will occur. And the issue is not unique to pathology. Situations in which there has been a tragic result, but not through the negligence of the physician, come up frequently in malpractice cases. The courts should be asking whether the physician is at fault. But often they ask who is in the best position to pay.”
Bierig offers this analogy to blinded review of slides in cytopathology malpractice cases: Where the police want a witness to identify a suspected perpetrator, they don’t just show the suspect to the witness and ask whether that suspect committed the crime. Rather, they place the suspect in a lineup with several other persons to see whether the witness can make the identification. Similarly, “an expert witness shouldn’t be given a slide that he or she knows has been misread and then asked to opine whether it has been misread. Instead,” Bierig says, “the expert witness should be given numerous slides, most of which are negative, and then asked to identify which of them, if any, are positive.”
Medical practitioners view things differently than lawyers and courts do, Dr. Crothers points out. “Speaking as a pathologist and a scientist, we wanted to provide a framework in which both opposing parties could make a decision as to whether a test was worth going to court over. It can benefit both parties, actually.”
“If you had a case under consideration for litigation in your lab and all the observers came back and called it high-grade epithelial lesion, then I think your defense attorney is going to have a different approach to that case than if it showed there were answers all over the field or most people called it normal,” Dr. Crothers says.
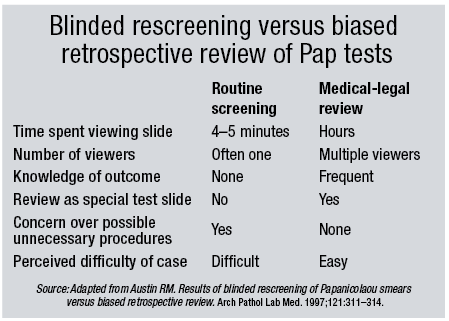
The court seemed to think the College was forcing a guideline on the court process. But that wasn’t the intent, she contends. “The intent was to inject a bit of reality into the whole process and raise awareness of the subjectivity of Pap test interpretation. Expert witnesses are biased through prior knowledge of the outcome and have an inherent conflict of interest if they are paid for their answers.”
In the appeals record, she notes, there are some interesting facts. “Under normal circumstances a cytotechnologist would take at most 10 minutes to screen a slide, but expert witnesses often will take two or three times that long. In this case, there were four slides but the expert took 90 minutes to review them. Now some photography might have accounted for some of that, but we don’t know that. It may mean that the cells perhaps weren’t so easy to find and you really had to scrutinize the slides to find them.”
Where a conscientious individual is doing his or her job and going at a routine clip, would that person reasonably be expected to find the one or two cells? “I don’t know, but that’s kind of where the question lies from a scientific point of view,” Dr. Crothers says.
Since the 1990s, the malpractice litigation environment has changed substantially, she notes. “The primary reasons are that we’ve introduced a lot of automation, and we’ve essentially cleaned up the procurement and preparation process.” About 95 percent of laboratories now use liquid-based cytology, which prevents the air-drying artifact and some of the preparation problems that used to arise, she says. “It allows for a more monolayer, even distribution of cells across the slides so they’re a little easier to interpret.” However, she adds, blood or inflammation are among factors that can still cause a problem.
Automated screening instruments help greatly. “It’s very common in large labs now to have an instrument preselect the fields of view, the potentially abnormal areas on a slide for the first observer, then the cytotechnologist reviews those to decide whether the slide is abnormal and needs complete screening, or lacks anything significant and can be signed out as negative.” Those instruments are finely tuned to high-grade epithelial lesions, “and that helps for those cases where there may be very few cells that are characteristic.”
The introduction of HPV testing has wrought other improvements in identifying high-risk patients, Dr. Crothers points out. “It has allowed cytology to take this big atypical squamous cell category that we’ve had and use an additional test, HPV testing, to triage those patients either to colposcopy or a repeat test in a year. And that’s been helpful because in the past, clinicians weren’t always sure what to do with that atypical squamous cell category, and patients might be over-screened and end up with multiple Paps, multiple biopsies and excisions, or they might get under-screened and possibly lost for follow-up.”
There are still so many other factors that the false-negative rate may not have decreased at all, however. “In fact, it probably hasn’t, but now we are able to more effectively get patients in the system with a clinical algorithm that outlines how they should be properly followed. With clinicians following that, we have a lot less opportunity to miss somebody who has a significant lesion.”
Bierig envisions three steps that the College might take in the wake of the Adams v. LabCorp decision. “First the guidelines should be basically neutral, and not suggest that there be a different approach for defendants than for plaintiffs. The College needs to make clear that whatever it believes to be the right approach to review of cytopathology slides in connection with litigation applies equally to experts for all parties to the case. I think that that was the intent, but it may not have been expressed as clearly as it could have been.”
Second, the College may want to include an explanation of the reasons for the guidelines. “If a court concludes, as this court did, that particular guidelines are nothing more than an effort to protect negligent physicians from liability, then the court will, of course, completely discount that effort. On the other hand, if the entity that issues guidelines explains what it is trying to do and why, I think the guidelines have a much better chance of being accepted by the court. It is, of course, impossible to know, but one wonders what this court would have done had the College’s guideline included a thoughtful explanation for the positions taken therein.”
Third, in general, any medical society guidelines should be reviewed no less often than every three years to ensure that the entity that issued them still agrees with them, and to modify them to reflect changes in science or medicine that may have occurred, Bierig recommends. “You want to make sure your guidelines reflect advances in the state of the art—as well as what the medical society thinks is right.”
[hr]
Anne Paxton is a writer in Seattle.
![]()