 The chapter in Autopsy Performance & Reporting titled “Pregnancy-Related Death and the Autopsy Examination” is written by Cynthia Schandl, MD, PhD, of Medical University of South Carolina. Here from that chapter is an excerpt (published without references) on the hepatic system. Dr. Schandl’s chapter also covers the cardiovascular, respiratory, hematopoietic, urogenital, and endocrine systems, as well as the gastrointestinal tract and skin and connective tissue.
The chapter in Autopsy Performance & Reporting titled “Pregnancy-Related Death and the Autopsy Examination” is written by Cynthia Schandl, MD, PhD, of Medical University of South Carolina. Here from that chapter is an excerpt (published without references) on the hepatic system. Dr. Schandl’s chapter also covers the cardiovascular, respiratory, hematopoietic, urogenital, and endocrine systems, as well as the gastrointestinal tract and skin and connective tissue.
The position of the liver is shifted in a superior posterior direction because of the enlarging uterus. The liver histology and mass do not normally change with pregnancy; however, a hepatic production of circulating proteins is altered in quantity. Specifically, factors VII, VIII, IX, and X and fibrinogen are significantly elevated. Along with these alterations, the placental tissues contain thromboplastin, which activates factor VII and can produce fibrin in approximately 12 seconds. This is extremely useful to the woman at delivery, who must be able to deliver the infant and the placenta without excessive blood loss. Measured alkaline phosphatase is also elevated by as much as 200%. Most of this elevation is due to the placental alkaline phosphatase isoenzyme. Other changes include a decrease in serum albumin, total protein, and g-globulin, and an increase in serum cholesterol (200%) and a-globulin and b-globulin (slight). Levels of antithrombin III (inactivator of factors II, IX, X, XI, and XII) and protein C (inactivator of factors X and XIII) and its cofactors, protein S and thrombomodulin, do not appear to be increased in tandem, thus contributing to a hypercoagulable state.

Fig. 1. Pulmonary saddle thromboembolism 1 day status post caesarian section. A. (above) Gross. B. (below) Microscopic. Other risk factors included morbid obesity. (Hematoxylin-eosin, low power.)
Embolic events account for the majority of deaths in the puerperium. Because the majority of embolic events involve thrombosis, the entity will be described here. Air and amniotic fluid embolism have been discussed (see “Cardiovascular System”). Thromboemboli must always be considered in the peripartum autopsy because of the aforementioned hypercoagulable state. Other risk factors during the pregnancy and postpregnancy state include caesarian section, maternal age of 35 years or older, obesity, and additional chronic conditions. Heyl et al recently reviewed all available cases in the states of Florida and Virginia between 1999 and 2007. They identified 46 deaths associated with pulmonary thromboembolic disease, which equated to a mortality ratio of 1.6 per 100,000 live births. Only about one-fifth of the women demonstrated symptoms in the preceding days before death; these symptoms included shortness of breath, leg or chest pain, leg weakness, and/or tachycardia.

Fig. 1, B
If pulmonary thromboembolism is suspected, the heart and lungs may be removed en bloc in order to follow the circulation through the heart into the pulmonary arteries, allowing better exposure of any pathology. With the bloc anterior surface facing the prosector, the direction of the blood flow can inform the dissection—first into the right atrium, then along the lateral wall of the right ventricle to its apex and up the anterior-medial aspect of the right ventricle into the pulmonary arterial circulation. The prosector should remain alert while cutting through any coronary arteries in order to identify dissection. If an embolus is not seen at the bifurcation (“saddle”; Fig. 1, A) of the main pulmonary trunk or proximal pulmonary arteries, the pulmonary arterial system should be dissected both lengthwise and by cross-section in order to assess for thromboembolism to the mid and peripheral pulmonary circulation. Multiple sections should be submitted for histologic examination. Grossly, if a thromboembolus is identified, a section taken through a region attached to the pulmonary intimal wall (if present) is ideal. A thromboembolus should demonstrate the microscopic features of a thrombus, with lines of Zahn appreciated (Fig. 1, B); however, prominence of this feature varies with coagulation status. If pulmonary thromboembolism is considered the cause of death and no significant comorbidities are present, at least 50% occlusion of the total pulmonary arterial vasculature should be apparent. In addition, dissection of the deep veins of the legs and the pelvic veins may be pursued in order to support the origin of the thrombosis.
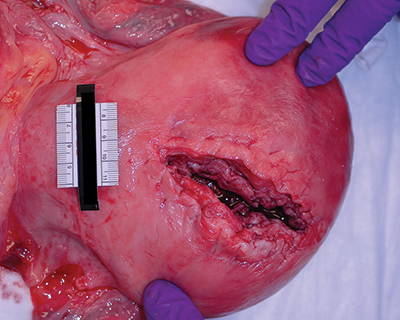
Fig. 2. Uterine rupture. A woman with an intrauterine pregnancy of estimated gestational age 32 weeks was found unresponsive at home. Resuscitation efforts were made. At the hospital, a caesarian section procedure was begun, and approximately 1500 mL of blood were evacuated from the peritoneal cavity. The viable baby was delivered and the uterus sutured, but the mother did not regain signs of life despite fluid and blood product provision and continued advanced life-support measures.
Obstetric hemorrhage was the leading cause of maternal mortality in the United States for the years 1998 to 2005 by a small margin. In addition, the majority of deaths from hemorrhage are considered preventable upon review. The number of women experiencing hemorrhage increased during the interval, and, in 2006, 2.9% of births were complicated by maternal hemorrhage, with the increase found to be secondary to increased incidence of uterine atony. Death from hypovolemic shock can occur if hemorrhage is not adequately identified or controlled in the peripartum period. A ruptured splenic artery aneurysm, spontaneous hepatic rupture, postpartum uterine atony, abruptio placentae, placenta previa, ruptured ectopic pregnancy, uterine rupture (Fig. 2), postpartum uterine inversion, or any disruption in the tenuously balanced coagulation cascade can lead to catastrophic blood loss. Uterine atony has been cited as the most common cause (79%; N=25,654 women with postpartum hemorrhage), and numerous independent risk factors are identified, including age (<20 years or 40 or more years), caesarian section with labor, hypertensive diseases of pregnancy, chorioamnionitis, polyhydramnios, multiple gestation, antepartum hemorrhage, and retained placenta. In addition, multiple etiologies lead to the common pathway of DIC and consequent hemorrhagic shock. These include sepsis, retained dead fetus, retained placental material (Fig. 3), amniotic fluid embolism, intravascular hemolysis, pregnancy-induced hypertension, placental abruption/disruption, and trauma. Any suture lines, vaginal or cervical lacerations, and the uterus itself should be examined in an attempt to locate the source of the hemorrhage. If bleeding was clinically oozing or diffuse, thrombotic microangiopathies of pregnancy should be suspected. In these cases, a specific site of bleeding may not be elicited upon examination. Such cases include thrombotic thrombocytopenic purpura/hemolytic-uremic syndromes (TTP/HUS), HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count), pregnancy-induced hypertension, acute fatty liver of pregnancy, antiphospholipid antibody syndromes, systemic lupus erythematosus/lupus vasculitis, or DIC. Fibrin thrombi, characteristic of DIC, can be identified or confirmed with a Weigert stain, which stains fibrin pink. Renal glomerular capillary loops are a common place to find these thrombi. Untreated, the mortality is high. In addition, a multitude of hereditary coagulopathies—including activated protein C resistance, factor V gene mutations, prothrombin 20210 G→A mutation, and hyperhomocysteinemia—may contribute to a poor outcome resulting from thrombotic events after peripartum hemorrhage. The likelihood of thromboembolism may also increase. Uncontrolled hemorrhage may result from complications of von Willebrand disease and, to a lesser extent, inherited or acquired hemophilias

Fig. 3. Retained placental material. Decidualized endometrium surrounds placental villi, consistent with retained placental materials. (10×, hematoxylin-eosin stained.)
TTP should be considered in the maternal patient suffering from a coagulation disorder. If possible, a premortem peripheral blood smear should be examined and the clinical workup reviewed. If the diagnosis was considered clinically—microangiopathic hemolytic anemia, altered mental status, renal failure, thrombocytopenia, fever—a hemapheresis or hematology consultation note might be available for postmortem review. Platelet thrombi may be more easily visualized if an immunostain for factor VIII is employed because this factor binds to the platelet surface. In the renal glomerulus, the lesions are more likely to be at the vascular pole. A factor VIII immunostain will also highlight the endothelial surface.
Hepatic diseases specific to the pregnant patient include acute fatty liver of pregnancy (AFLP), the HELLP syndrome, spontaneous hepatic rupture, hyperemesis gravidarum, and intrahepatic cholestasis of pregnancy. Although hyperemesis gravidarum and intrahepatic cholestasis rarely result in mortality, AFLP and HELLP may lead to maternal death.
AFLP often occurs with pregnancy-induced hypertension, although its incidence is only approximately 1 in 10,000 to 20,000 pregnancies. Still, there is up to 45% fetal death and up to 10% maternal death when the disease does manifest. Histopathologic findings include microvesicular steatosis and, later, cholestatic lobular hepatitis. If AFLP is suspected, histologic evidence may include a frozen section of liver stained with oil red O or a similar lipophilic compound because the fat is removed during routine specimen processing and will only be evident by clear round spaces in the tissue. Extramedullary hematopoiesis and megamitochondria may also be present. In HELLP syndrome, fibrin deposition and intrahepatic periportal hemorrhage are seen. Both AFLP and HELLP syndrome can lead to multiple complications, although the maternal mortality rate is significantly higher in AFLP. The mortality rate has been affected by improvements in supportive care measures and for AFLP is reported as 7% to 18% and for HELLP as 1% to 25%. Up to 70% of women with AFLP and only approximately 15% of women with HELLP will suffer from DIC and acute renal failure. It is interesting to note that women with AFLP or HELLP during pregnancy are more likely to have fetuses with a mitochondrial fatty acid beta-oxidation defect due to deficiency of LCHAD (long-chain 3-hydroxyacyl-coenzyme A dehydrogenase). Thus, if the infant survives, it is important to convey this diagnosis to the pediatric care team in order to provide proper care while testing for inborn errors of metabolism is underway. On the other hand, it was observed that maternal liver disease was 18.1-fold higher when the fetus was affected by a fatty acid oxidation defect. Spontaneous hepatic rupture is rare, with an incidence of approximately 1 in 100,000 pregnancies. The etiology is unclear but may be due to infarction from fibrin deposition in combination with hypovolemia. Minor external trauma, possibly even uterine contractions, may play a role. Hepatic rupture may also occur as a complication of other hepatic diseases during pregnancy, such as HELLP and AFLP. Maternal mortality from hepatic rupture approaches 50%.