Karen Lusky
July 2019—Most fresh blood in lung wedge biopsies is artifact, but when it’s diffuse alveolar hemorrhage, the pathologist must call the clinician because DAH patients can go downhill fast. Maxwell L. Smith, MD, a consultant in the Department of Laboratory Medicine and Pathology at Mayo Clinic Arizona and associate professor, Mayo Clinic School of Medicine, shared that pearl from one of the 10 consultation cases he and Brandon T. Larsen, MD, PhD, co-presented in their CAP18 session on diagnosing interstitial lung disease. Their discussion of two of those cases follows.
Second of two parts
A 51-year-old female had blood-tinged sputum, a sore throat, and cough in the prior few weeks. The woman’s chest x-ray displayed “sort of a symmetric, almost butterfly-shaped set of opacities,” said Dr. Larsen, a senior associate consultant in the same department at Mayo Clinic Arizona and associate professor, Mayo Clinic School of Medicine. (For this case, he was acting as the original pathologist; Dr. Smith would then enter and explain the diagnostic pitfall and how to avoid it. In the next case, they reverse roles.) Now Dr. Larsen had wedge biopsies to examine (Fig. 1). “At low power, I think the first thing that strikes me is there is a bunch of blood in the tissue, but that’s probably just from the surgeon. I don’t see fibrosis.”
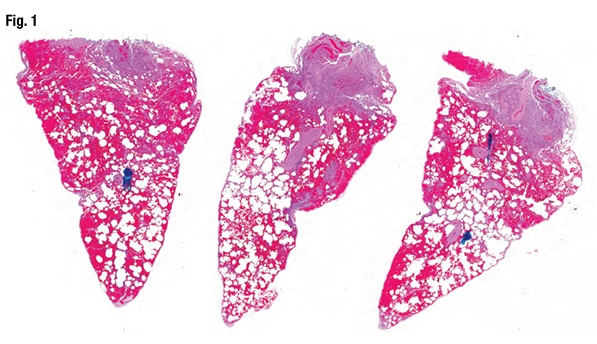 At somewhat higher power, Dr. Larsen spotted a fairly fresh thrombus in a small pulmonary artery. He went on to identify other thrombi or thromboemboli, noting it was difficult to know which. “There’s definitely organizing pneumonia; I do see that. So organizing pneumonia with acute thromboemboli.” He wasn’t entirely sure “how to put all of that together,” he said, and since it was a Friday afternoon, the case had to be sent out right away. His question for a consultant: “Is there a relationship between the pulmonary artery changes and the organizing pneumonia?”
At somewhat higher power, Dr. Larsen spotted a fairly fresh thrombus in a small pulmonary artery. He went on to identify other thrombi or thromboemboli, noting it was difficult to know which. “There’s definitely organizing pneumonia; I do see that. So organizing pneumonia with acute thromboemboli.” He wasn’t entirely sure “how to put all of that together,” he said, and since it was a Friday afternoon, the case had to be sent out right away. His question for a consultant: “Is there a relationship between the pulmonary artery changes and the organizing pneumonia?”
“We’ll talk about that,” Dr. Smith said. He recommended going back and looking at subtle findings on the biopsy. Hemosiderin-laden macrophages “are obscured by all that blood, but you can see these little rust-colored macrophages that are sometimes challenging to differentiate from respiratory bronchiolitis in a smoker.” (Fig. 2). Compared with smoker macrophages, hemosiderin-laden macrophages should contain larger granules with a more “refractile appearance.” Both will be iron positive, so there’s no need for an iron stain.
He noted a small amount of fibrin in the area of the organizing pneumonia and many neutrophils within the interstitium (Fig. 3). When pathologists see subtle neutrophils within what is otherwise an organizing pneumonia or acute fibrinous lung injury process, they should begin thinking about diffuse alveolar hemorrhage, Dr. Smith advises.
Showing an image of fibrin embedded with fresh red blood cells in the patient’s airspace (Fig. 4), Dr. Smith asked the audience members to imagine momentarily they are the surgeon obtaining the biopsy. And all of the blood in the airspace is related to the procedure. “Would you expect to have red blood cells embedded within fibrin? Not at all, right? Because these red blood cells embedded within the fibrin suggest that they are there when the fibrin is being formed around it.”
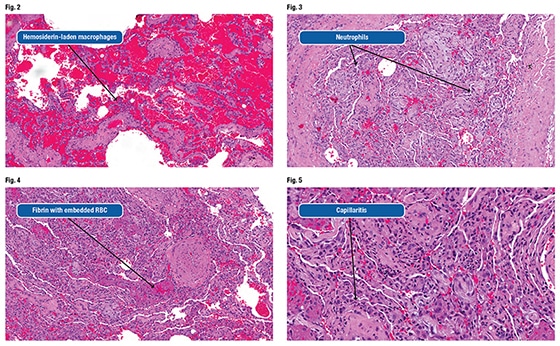 Dr. Smith also pointed out microvascular capillaritis, which is the lesion of diffuse alveolar hemorrhage (Fig. 5). “We call DAH a vasculitis,” he said. In an interview with CAP TODAY, Dr. Larsen noted that when most pathologists hear that clinicians are worried about vasculitis, they are looking for an obvious, inflamed large vessel, but in the lung, capillaritis is a far more frequent manifestation of that disorder, and very subtle.
Dr. Smith also pointed out microvascular capillaritis, which is the lesion of diffuse alveolar hemorrhage (Fig. 5). “We call DAH a vasculitis,” he said. In an interview with CAP TODAY, Dr. Larsen noted that when most pathologists hear that clinicians are worried about vasculitis, they are looking for an obvious, inflamed large vessel, but in the lung, capillaritis is a far more frequent manifestation of that disorder, and very subtle.
The consult diagnosis was “acute and organizing diffuse alveolar hemorrhage with capillaritis.” The comment was as follows: “The differential diagnosis for DAH encompasses numerous immune-mediated diseases, including antineutrophil cytoplasmic antibody-associated vasculitis. Correlation with a complete serological workup is suggested. This patient might benefit from immune suppression and immune modulation.”
 The ball is largely back in the clinicians’ court, Dr. Smith said. “They need to do the serology workup and figure out exactly what immune-mediated process it is.” The problem, though, was that Mayo Clinic didn’t receive the consult until the following Tuesday because the pathologist shipped it on Friday. And on that same Friday, the patient could not be extubated after surgery and was admitted to the ICU where she had massive hemoptysis on Saturday morning. So the clinician initiated empirical immunosuppression. The woman remained in critical condition, which Dr. Smith said is why DAH is an important diagnosis not to be missed. He also emphasized that these cases need immediate review.
The ball is largely back in the clinicians’ court, Dr. Smith said. “They need to do the serology workup and figure out exactly what immune-mediated process it is.” The problem, though, was that Mayo Clinic didn’t receive the consult until the following Tuesday because the pathologist shipped it on Friday. And on that same Friday, the patient could not be extubated after surgery and was admitted to the ICU where she had massive hemoptysis on Saturday morning. So the clinician initiated empirical immunosuppression. The woman remained in critical condition, which Dr. Smith said is why DAH is an important diagnosis not to be missed. He also emphasized that these cases need immediate review.
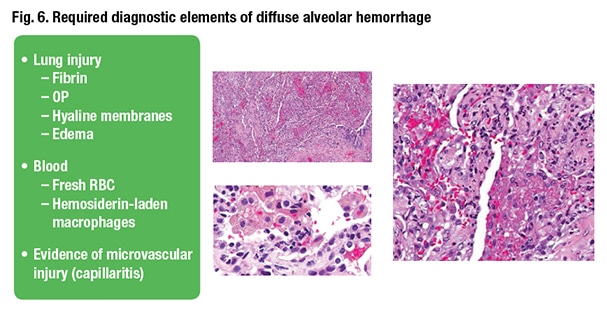
What histological criteria does the pathologist have to see on the biopsy to diagnose DAH? The first, Dr. Smith said, is indication of acute lung injury. “If you do not have any lung injury, you do not have diffuse alveolar hemorrhage. I don’t care how many hemosiderin-laden macrophages you see.” The second criterion is histologic evidence of blood where it shouldn’t be in the airspaces. That can be hemosiderin-laden macrophages or fresh red blood cells. Last is evidence of microvascular injury or capillaritis (Fig. 6).
 The textbooks advise looking for capillaritis and, if not present, then reporting “diffuse alveolar hemorrhage without capillaritis,” Dr. Smith continued. “But how does the blood get there? There’s microvascular injury there somewhere; you are just not seeing it. So the clue is to go to the areas of fibrin because the fibrin is telling you, ‘Hey look, there is microvascular injury here.’” That’s the reason fibrin is in the airspaces, so that’s where pathologists should focus their investigation in capillaritis.
The textbooks advise looking for capillaritis and, if not present, then reporting “diffuse alveolar hemorrhage without capillaritis,” Dr. Smith continued. “But how does the blood get there? There’s microvascular injury there somewhere; you are just not seeing it. So the clue is to go to the areas of fibrin because the fibrin is telling you, ‘Hey look, there is microvascular injury here.’” That’s the reason fibrin is in the airspaces, so that’s where pathologists should focus their investigation in capillaritis.
They can be subtle, but look for neutrophils with associated enlarged and reactive-looking endothelial cells in the interstitium and in the capillary network, Dr. Smith advises. “It’s analogous to endocapillary proliferation in the glomerulus. That’s what you are looking for in the capillary network of the airspace.”
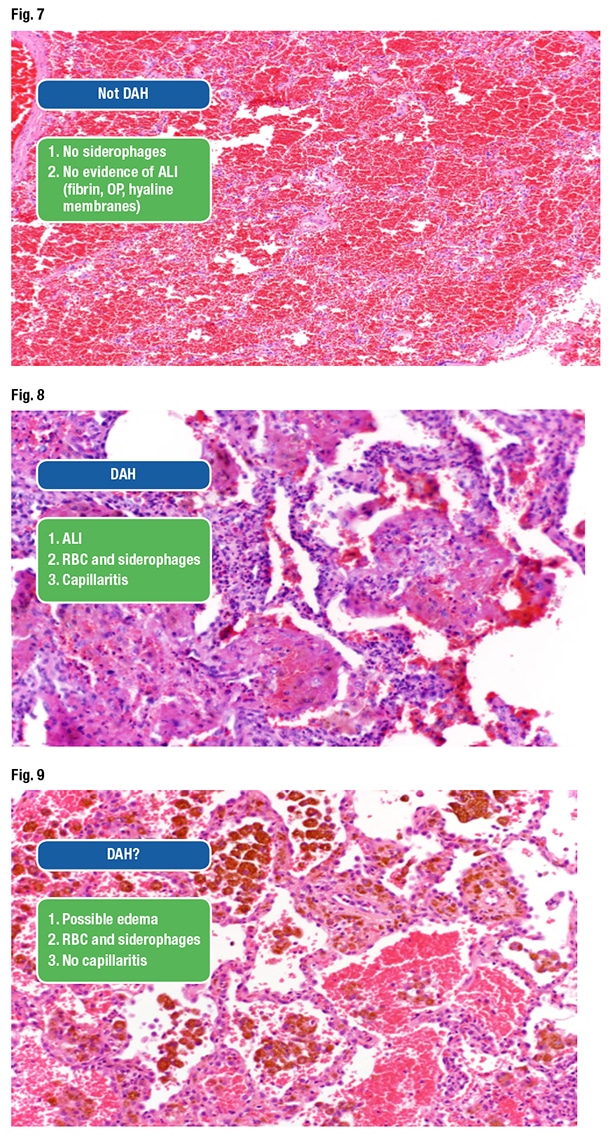
Dr. Smith presented an image and asked the audience if it was DAH (Fig. 7). “You have red blood cells. Is there any acute lung injury here? Absolutely not,” and thus it is not diffuse alveolar hemorrhage. There are no hemosiderin-laden macrophages either. He shared another image, which was DAH (Fig. 8). “Easy, right? Acute lung injury, fibrin, capillaritis, red blood cells entrapped within the fibrin, hemosiderin-laden macrophages. That’s diffuse alveolar hemorrhage.”
The next image was more challenging, he said, due to the red blood cells, hemosiderin macrophages, and “clear-cut evidence” of bleeding in the airspaces (Fig. 9). Yet there’s no definitive acute lung injury apart from perhaps slight edema within the alveolar walls. “This is one where I would keep searching because I know there is a lot of bleeding coming from somewhere. But what else can give you a lot of hemosiderin in your lung like this?” Congestive heart failure, he said. “We don’t usually get those biopsies because they can usually diagnose that clinically.”
An attendee asked about the significance of the big vessel thrombus in the patient’s case and if it is compatible with DAH. Dr. Smith replied that, in his view, organizing thrombi in pulmonary arteries is a feature seen in the setting of acute lung injury. “In addition to OP and fibrin, it is common to have organizing thrombi in the pulmonary arteries. It’s a secondary process to the acute lung injury happening,” he said, “and it is not related to the DAH.”
 Dr. Smith presented another case in which a 55-year-old man had shortness of breath, cough, weight loss, and fevers for a year. The patient used antibiotics and steroids without improvement. Imaging studies revealed infiltrates and nodules more so in the right lung than the left.
Dr. Smith presented another case in which a 55-year-old man had shortness of breath, cough, weight loss, and fevers for a year. The patient used antibiotics and steroids without improvement. Imaging studies revealed infiltrates and nodules more so in the right lung than the left.
In the role of the original pathologist in this case, Dr. Smith inspected a wedge biopsy of the patient’s lung at high power and identified nonspecific interstitial pneumonia, organizing pneumonia, fibrin with an eosinophil, an airway with chronic bronchiolitis, perhaps an interstitial granuloma, a vessel with perivascular infiltrates, a non-necrotizing granuloma, and a possible giant cell.
“This is all one slide of one case. Diagnosis deferred,” the original pathologist said in comments to the Mayo Clinic consulting pathologist, adding, “This biopsy shows almost all of the patterns of lung injury that have ever been described. I have no idea what to suggest in this case.” Included were the imaging studies.
 Dr. Larsen displayed the CT scan of the patient’s lungs (Fig. 10). “Even I can recognize there’s something wrong in the right lung,” he said. “This is just diaphragm, of course, but there is some sort of patchy, ground glass infiltrate in the right lung and, looking a little more closely, there probably is even some of the same process going on in the left lung, just a lot more subtle.” The problem appears to be bilateral.
Dr. Larsen displayed the CT scan of the patient’s lungs (Fig. 10). “Even I can recognize there’s something wrong in the right lung,” he said. “This is just diaphragm, of course, but there is some sort of patchy, ground glass infiltrate in the right lung and, looking a little more closely, there probably is even some of the same process going on in the left lung, just a lot more subtle.” The problem appears to be bilateral.
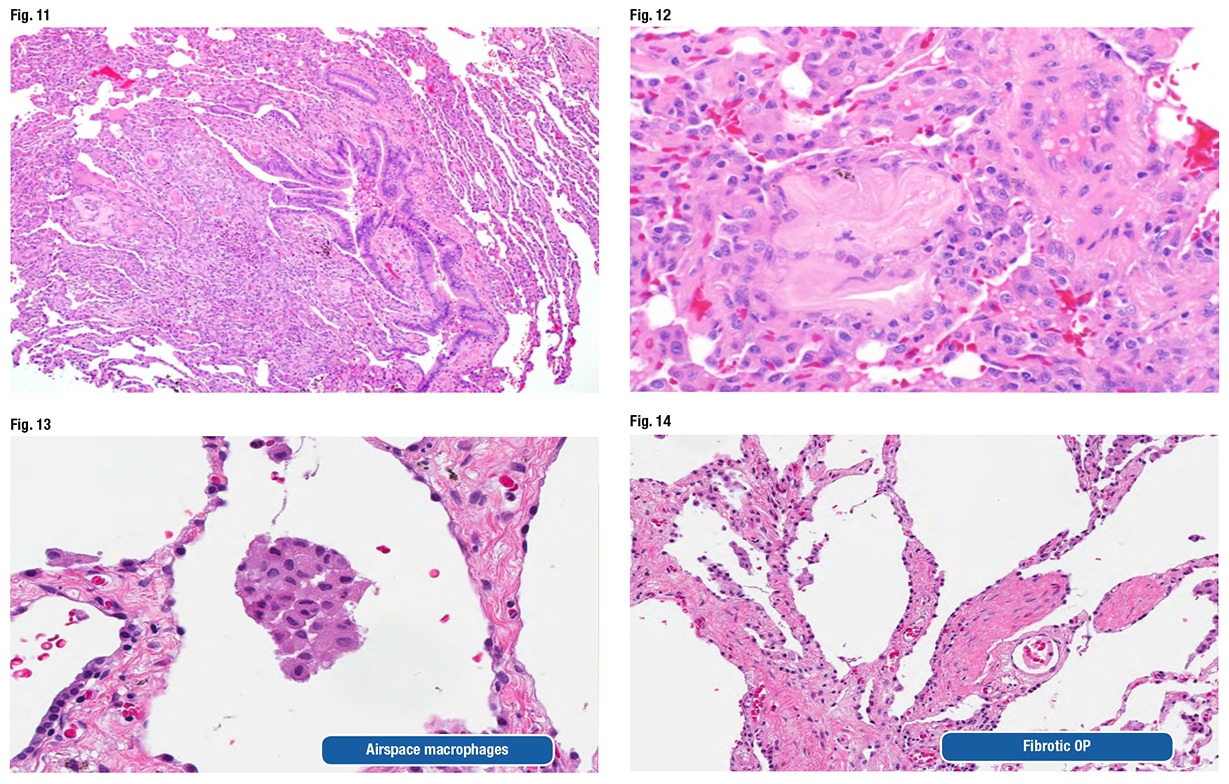
 He also looked at the biopsy and confirmed everything the original pathologist found. Then he recognized the culprit. One of the fragments of aspirated food looks “almost like a light pink letter S that has a little dark purple eye in the middle of it,” at about nine o’clock in the periphery of the biopsy at low power, Dr. Larsen told CAP TODAY (Fig. 11). Another fragment shown at high power is the “irregular pink blob that’s just to the left of center.” (Fig. 12).
He also looked at the biopsy and confirmed everything the original pathologist found. Then he recognized the culprit. One of the fragments of aspirated food looks “almost like a light pink letter S that has a little dark purple eye in the middle of it,” at about nine o’clock in the periphery of the biopsy at low power, Dr. Larsen told CAP TODAY (Fig. 11). Another fragment shown at high power is the “irregular pink blob that’s just to the left of center.” (Fig. 12).
“This amorphous eosinophilic stuff is not really recognizable as food anymore, but this, in our experience at least, tends to be one of the most common types of fragments encountered with people who aspirated,” Dr. Larsen said. The fragments “can be really tricky, and if you are going quickly, you will gloss over this, thinking it’s just some sort of fibrous tissue that is sort of a little weird in the interstitium, but in fact that is aspirated foreign material.”
 The patient’s consult diagnosis was diffuse aspiration bronchiolitis and pneumonitis. Dr. Larsen presented Table 1 from an article by a Mayo Clinic pulmonologist and colleagues (Hu X, et al. Chest. 2015;147[3]:815–823). The syndromes include problems of the airways, Dr. Larsen said, pointing out diffuse aspiration bronchiolitis, which chronically can lead to bronchiolitis obliterans syndrome.
The patient’s consult diagnosis was diffuse aspiration bronchiolitis and pneumonitis. Dr. Larsen presented Table 1 from an article by a Mayo Clinic pulmonologist and colleagues (Hu X, et al. Chest. 2015;147[3]:815–823). The syndromes include problems of the airways, Dr. Larsen said, pointing out diffuse aspiration bronchiolitis, which chronically can lead to bronchiolitis obliterans syndrome.
All of the risk factors listed in Table 2 (Hu X, et al. Chest. 2015;147[3]:815–823) should be apparent, Dr. Larsen said: dysphagia, altered mental status owing to excessive alcohol, neurologic disorders, esophageal disorders, vomiting, GERD, etc. “Lots of things can lead to aspiration,” he said. “So always include aspiration in your differential for airway-centered inflammatory or fibrotic processes.”
The Chest article says “the presence of foreign material on the lung biopsy can be easily overlooked by pathologists, resulting in misdiagnosis.”
“This is a clinician’s statement,” Dr. Larsen said. “A little presumptive, right? We are pathologists. How can you tell us that we have missed food in our biopsies?” But he said the statement is completely accurate. Some of the food fragments actually polarize. “So tossing your polarizing filter on your scope and taking a second look sometimes can be helpful not to miss stuff, although not everything that is aspirated polarizes.”
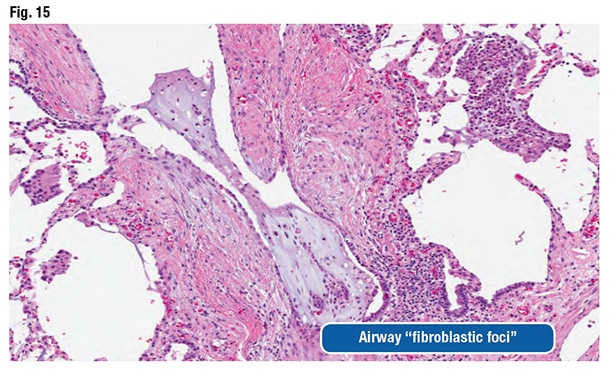
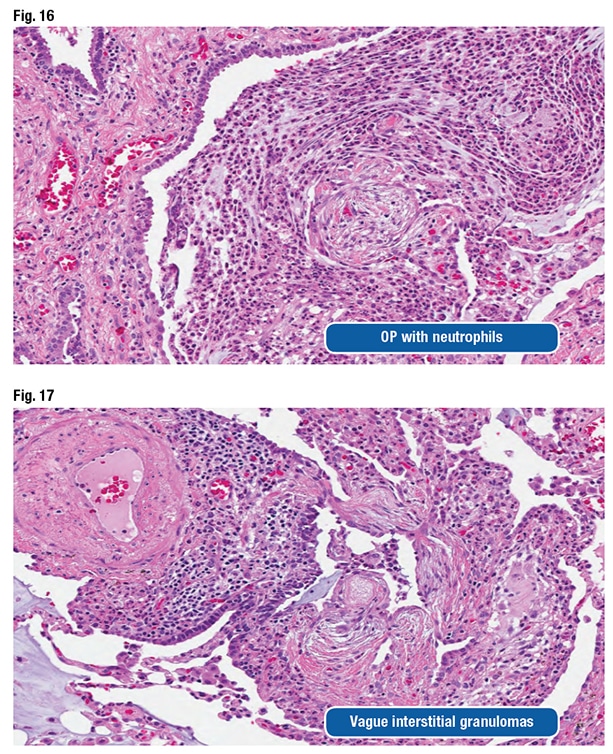
The pathologic features can be nonspecific and variable, as they were in this case, Dr. Larsen said. Airspace macrophages can appear foamy and even resemble drug-reaction macrophages (Fig. 13). “You can see organizing pneumonia” and sometimes fibrosing organizing pneumonia (Fig. 14). Fibroblast foci might be observed around the airways. “They look like fibroblast foci in a fibrotic ILD like a UIP [usual interstitial pneumonia] pattern-type fibroblast focus, but they are in the wrong spot,” he said. “They are around the airways.” (Fig. 15).
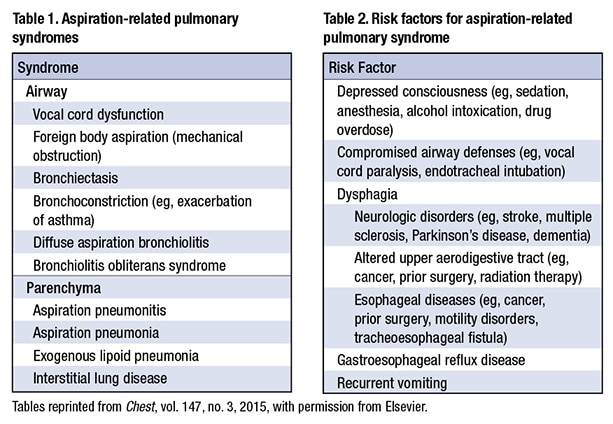 The pathologist could also see acute and chronic bronchiolitis, vasculopathy, vague interstitial granulomas, and organizing pneumonia with neutrophils. “You sort of wonder if that has secondary infection related to aspiration,” he said (Figs. 16 and 17).
The pathologist could also see acute and chronic bronchiolitis, vasculopathy, vague interstitial granulomas, and organizing pneumonia with neutrophils. “You sort of wonder if that has secondary infection related to aspiration,” he said (Figs. 16 and 17).
Dr. Larsen presented an image of prominent airway disease at low power (Fig. 18). “When I see abnormalities like that, aspiration gets really high on my differential. In this particular case, only a single fragment of aspirated vegetable material was seen, even though there were all those other changes,” he said, referring to a second image (Fig. 19). “You have got to search for this, but in an appropriate context, this is absolutely diagnostic of aspiration as the cause.”
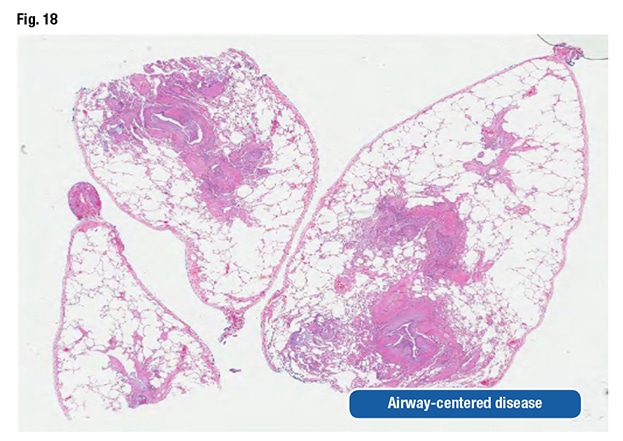 With airway-centered disease, the pathologist has to consider aspiration. “Even if you can’t find food and prove it, you still need to suggest it to the clinicians because they can actually do something about it.”
With airway-centered disease, the pathologist has to consider aspiration. “Even if you can’t find food and prove it, you still need to suggest it to the clinicians because they can actually do something about it.”
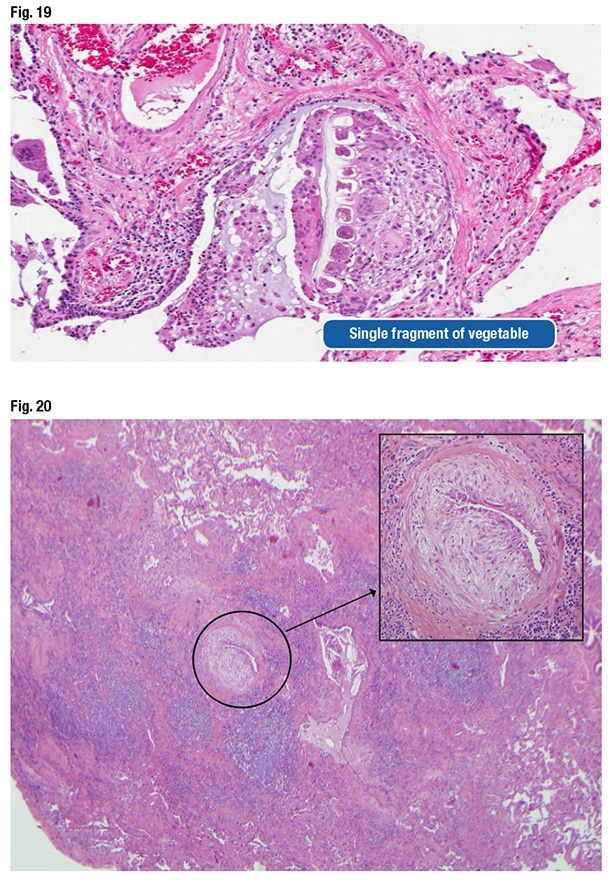 Dr. Larsen also shared a case they became aware of when a Mayo Clinic clinician asked them to look at a wedge biopsy of a patient diagnosed with UIP a decade earlier. The clinician said it didn’t make sense that the patient has UIP and is still alive. “So we re-reviewed the case,” Dr. Larsen said, pointing out structures in the biopsy that look like fibroblast foci (Fig. 20). “You can see why somebody might have started to think about UIP, because there’s scar here, but also all of this centrilobular inflammatory injury.” However, “this fibroblastic proliferation is not at the front of progressive scar. It’s actually occurring within an airway. It’s submucosal fibroblastic proliferation that, in this case, demonstrated aspirated food particles that had been missed initially.” The patient had chronic aspiration pneumonia with scar formation imitating UIP. (No food fragments are in the photo, Dr. Larsen says, but they can be found elsewhere in the biopsy.)
Dr. Larsen also shared a case they became aware of when a Mayo Clinic clinician asked them to look at a wedge biopsy of a patient diagnosed with UIP a decade earlier. The clinician said it didn’t make sense that the patient has UIP and is still alive. “So we re-reviewed the case,” Dr. Larsen said, pointing out structures in the biopsy that look like fibroblast foci (Fig. 20). “You can see why somebody might have started to think about UIP, because there’s scar here, but also all of this centrilobular inflammatory injury.” However, “this fibroblastic proliferation is not at the front of progressive scar. It’s actually occurring within an airway. It’s submucosal fibroblastic proliferation that, in this case, demonstrated aspirated food particles that had been missed initially.” The patient had chronic aspiration pneumonia with scar formation imitating UIP. (No food fragments are in the photo, Dr. Larsen says, but they can be found elsewhere in the biopsy.)
Aspiration is not invariably unilateral, he cautions. “We are taught in medical school that people aspirate into the right lower lobe,” which is the quickest and easiest location for food to go when a person aspirates it into the lungs. “But remember, it sort of depends on how you sleep at night.” A person who sleeps on the left side isn’t going to aspirate into the right lower lobe; it will go into a different lobe. “And if you happen to roll in the middle of the night into a different position and then aspirate again, you are going to get aspiration involving some other spot.” Therefore, aspiration is often multifocal and can be bilateral.
Dr. Larsen reminded the audience that the patient in the case had “preferential involvement of the right side, which is interesting, and should make you think about aspiration, but there are bilateral abnormalities that should not dissuade you from suggesting that possibility.”
The pearl for the case: “Aspiration-induced injury may be more common than you think.” Dr. Larsen reported signing out a case one day earlier in which the pathologist identified the inflammatory process, the bronchiolitis, and the organizing pneumonia. “But they completely missed the fragments of aspirated food within that process that would have clinched that diagnosis had they recognized them. We see pathologists missing this almost on a daily basis in our consultation practice. It’s one of the more common problems that trip people up.”
Karen Lusky is a writer in Brentwood, Tenn. Part one was published in the June issue of CAP TODAY. Drs. Larsen and Smith will present all 10 of their cases at CAP19 in Orlando, Fla., Sept. 21–25.