Q. Many laboratories, including ours, stopped repeating critical values after literature favored the expediency of initial critical value results, given the precision of state-of-the-art instrumentation. Nurses and physicians in our system now prefer that we release critical values for inpatients to the LIS before achieving successful critical value verification/call/documentation, which can take considerable time. Is there expert opinion on or standard practice for the release of preliminary critical values (denoted as such in the LIS lab results field) pending subsequent technologist or technician verification and documentation?
A. Some of the confusion in reporting critical values can be obviated by recognizing that there are three distinct activities: 1) verifying the critical value, 2) releasing the critical value, and 3) notifying a responsible provider, which is often by phone and includes documenting “read back” for laboratory records.
The first step is to verify the critical value, which, depending on laboratory policy, may or may not require retesting of the sample. There is no regulatory requirement to retest a sample when a result falls within the laboratory-defined critical range. On the contrary, the improved analytical precision and reproducibility of modern analyzers has made retesting of samples largely unnecessary. This is particularly true for modern chemistry and hematology analyzers, which generate the majority of critical results. The analytical performance of the test method dictates the level of confidence in any value, whether the result obtained is normal, abnormal, or critical. Thus, repeating tests may be unnecessary from a quality perspective and may only delay the reporting of a critical test result. Although the time needed to repeat automated testing is relatively short, delayed critical result reporting can cause adverse events.
Releasing a critical value renders that laboratory result viewable to providers within the patient’s chart. The result may be released or transmitted to the laboratory information system or the electronic health record. If laboratory policy requires verification of critical results by repeating the test, then the first result should not be released until it is verified by retesting. Releasing the value and notifying the provider are independent actions that can be performed in any order. There is no requirement that a critical value be held (i.e. not released) until notification is completed. However, the standard practice for many laboratories is to release the result once they have both verified the result and completed notification. (See “Sequence of information flow in critical value reporting: standard.”) The main reason many laboratories do not release critical values until they have notified the provider is they are worried the technologist will forget to follow up and complete notification once the value is released when their initial attempt to notify is unsuccessful. Thus, the main reason to hold the result is operational and based on the limitations of computer systems that make documenting provider notification difficult once the result is released. The limitations can be traced to the way the LIS interacts with or is interfaced with the EHR and the processes laboratories use to report critical values.
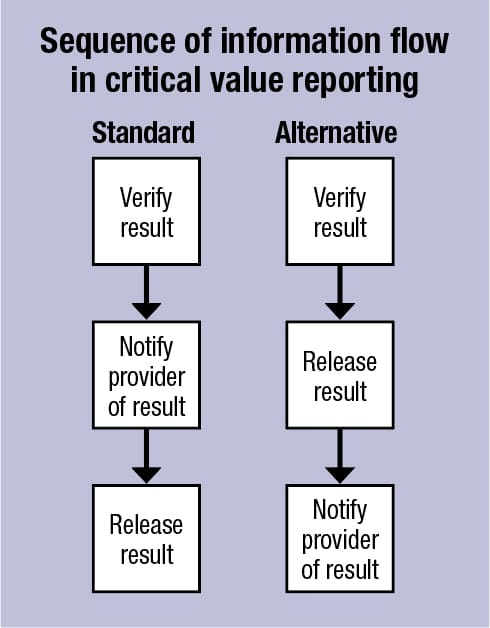 The practice of holding critical results until successful provider notification may not be in the best interest of patient care with the widespread adoption of the EHR, which has led to the rapid dissemination of and increased accessibility to patient information. An alternative flow of information may be more suitable (see “Sequence of information flow in critical value reporting: alternative”), in which the critical result is released after it is verified but before notification. This is analogous to instances that already occur in the standard model in which a laboratory releases a result to the EHR when it is unable to complete notification after one or more attempts. In the inpatient setting, released critical results are often recognized by a nurse, advanced provider, or another doctor before the ordering provider or a licensed caregiver is contacted. In either circumstance, the laboratory remains responsible for follow-up and verification of result receipt after the value is released and transmitted to the EHR. Follow-up systems can be developed within the EHR that help to close the communication loop and to ensure notification after the results are transmitted electronically.
The practice of holding critical results until successful provider notification may not be in the best interest of patient care with the widespread adoption of the EHR, which has led to the rapid dissemination of and increased accessibility to patient information. An alternative flow of information may be more suitable (see “Sequence of information flow in critical value reporting: alternative”), in which the critical result is released after it is verified but before notification. This is analogous to instances that already occur in the standard model in which a laboratory releases a result to the EHR when it is unable to complete notification after one or more attempts. In the inpatient setting, released critical results are often recognized by a nurse, advanced provider, or another doctor before the ordering provider or a licensed caregiver is contacted. In either circumstance, the laboratory remains responsible for follow-up and verification of result receipt after the value is released and transmitted to the EHR. Follow-up systems can be developed within the EHR that help to close the communication loop and to ensure notification after the results are transmitted electronically.
We recommend avoiding the use of the term “preliminary critical value” because once a value is released—whether preliminary or final—providers may act on that value. Again, the value should be verified before it is released, and there is no requirement that precludes releasing a critical result before successful notification. The most important rule for laboratories to follow is to have clear policies and procedures for critical value reporting that are approved by the laboratory medical director and hospital or health system committees, when applicable. Audits should be performed to ensure these procedures are followed and that they effectively provide critical results to caregivers in a timely and efficient manner.
- College of American Pathologists. GEN.43825 Result verification. In: Laboratory general checklist. Aug. 22, 2018.
- College of American Pathologists. COM.30000 Critical result notification. In: All common checklist. Aug. 22, 2018.
- College of American Pathologists. COM.30100 Critical result read-back. In: All common checklist. Aug. 22, 2018.
- Lehman CM, Howanitz PJ, Souers R, Karcher DS. Utility of repeat testing of critical values: a Q-Probes analysis of 86 clinical laboratories. Arch Pathol Lab Med. 2014;138(6):788–793.
- Valenstein PN, Wagar EA, Stankovic AK, Walsh MK, Schneider F. Notification of critical results: a College of American Pathologists Q-Probes study of 121 institutions. Arch Pathol Lab Med. 2008;132(12):1862–1867.
- Meier FA, Souers RJ, Howanitz PJ, et al. Seven Q-Tracks monitors of laboratory quality drive general performance improvement: experience from the College of American Pathologists Q-Tracks program 1999–2011. Arch Pathol Lab Med. 2015;139(6):762–775.
- Deetz CO, Nolan DK, Scott MG. An examination of the usefulness of repeat testing practices in a large hospital clinical chemistry laboratory. Am J Clin Pathol. 2012;137(1):20–25.
- Niu A, Yan X, Wang L, Min Y, Hu C. Utility and necessity of repeat testing of critical values in the clinical chemistry laboratory. PLOS One. 2013;8(11):e80663.
Peter L. Perrotta, MD
Medical Director of Clinical Laboratories, West Virginia University Hospital
Professor of Pathology and Chair, Department of Pathology, Anatomy, and Laboratory Medicine
West Virginia University School of Medicine, Morgantown
Chair, CAP Quality Practices Committee
Member, CAP Council on Scientific Affairs
Christopher M. Lehman, MD
Clinical Professor of Pathology, University of Utah School of Medicine
Medical Director of Clinical Laboratories, University of Utah Health Hospitals, Salt Lake City
Chair, CAP Standards Committee
Member, CAP Checklists Committee
Q. We are hoping to validate a procedure for the fixation, decalcification, and staining of bone marrow specimens including bone marrow core biopsy, a service we do not currently provide. Our problem is we will not be able to access fresh marrow specimens for our decalcification validation. Could you recommend an alternative tissue we could use to validate the preservation of tissue morphology and antigenicity after decalcification? We have several other tissue samples we could potentially access, including bone, gastrointestinal, dermatologic, and breast. Could any of these tissues be used? If not, what specimen do you recommend?
A. Consideration of effects of alternative fixation and decalcification protocols on morphology, antigenicity, and even DNA-RNA preservation is important in surgical and hematopathology. A variety of commercial and homebrew chemical decalcification methods are available and tend to differ widely between laboratories. In general, acid-based protocols are more deleterious to immunostaining.1,2 Testing and validation of such protocols are covered in CAP immunohistochemistry validation guidelines.3 Recommendation No. 8, from Table 3 of the CAP IHC guidelines, states: “If IHC is regularly done on decalcified tissues, laboratories should test a sufficient number of such tissues to ensure that assays consistently achieve expected results. The laboratory medical director is responsible for determining the number of positive and negative tissues and the number of predictive and nonpredictive markers to test.”3 The example of bone marrow biopsies discussed in the CAP IHC guideline encompasses the following: “To assess the influence of their decalcification procedure on IHC test results in bone marrows, laboratories should test a selected set of commonly ordered markers (eg, CD3, CD20, CD138) in a series of cases. The results may be correlated with expected results in routinely processed (control) tissues and with other applicable test results (eg, flow cytometry, IHC testing of lymph node in same patient).”3
Test tissue might include other bone specimens containing marrow elements together with other hematolymphoid tissues. Autopsy might offer another source of bone marrow biopsies, although it is not available to all laboratories. We find that tonsil, thymus, and spleen are available in many laboratories4; spleen with extramedullary hematopoiesis might provide an abundant source of non-lymphoid and hematopoietic precursor cells. Construction of multi-tissue block(s) could provide a substrate for testing of multiple relevant antibodies on paired decalcified and nondecalcified tissue with increased efficiency.
In an attempt to validate the direct effects of decalcification on antigens, we have incubated a commonly used immunohistochemistry protocol control tissue (i.e. tonsil tissue) in decalcification agents, followed by image analysis to quantify the effects.4 We also compared the effect of decalcification on breast tissue, obtained at reduction mammoplasty5; others have used excess breast tumor tissue.2,6,5 These may provide ancillary validation of the effects of decalcifying agents. Importantly, these studies showed that not all antigens from tonsil or breast tissue were affected in the same way as bone marrow. Further, demonstrating that decalcification does not impair CK7 staining would not translate to a CD61 stain. Thus, testing each antibody to be used in decalcified tissue would be considered best practice. In addition, it is critical that the tissue type to be used for validation, including those proposed here, include the cellular or disease process target for a given marker.
In summary, there is no easy way to provide optimal controls for all antigens used in IHC testing, and the individual laboratory should exercise diligence in characterizing the effects of decalcification on bone tissue.
- Arber JM, Arber DA, Jenkins KA, Battifora H. Effect of decalcification and fixation in paraffin-section immunohistochemistry. Applied Immunohistochemistry. 1996;4(4):241–248.
- Schrijver WA, van der Groep P, Hoefnagel LD, et al. Influence of decalcification procedures on immunohistochemistry and molecular pathology in breast cancer. Mod Pathol. 2016;29(12):1460–1470.
- Fitzgibbons PL, Bradley LA, Fatheree LA, et al. Principles of analytic validation of immunohistochemical assays: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2014;138(11):1432–1443.
- Shvartsbeyn M, Salama ME, Dewar R. Histologic evaluation of the effects of tissue decalcification on immunohistochemical markers in bone marrow specimen. 105th Annual Meeting of the USCAP; March 12–18, 2016; Seattle. Mod Pathol. 2016;29(Suppl2):504A.
- Hathuc VM, East E, Pang J, Jorns JM, Dewar R. A practical quality control procedure for decalcified bone specimens in evaluation of ER/PR/HER-2 immunohistochemistry. 106th Annual Meeting of the USCAP; March 4–10, 2017; San Antonio. Mod Pathol. 2017;30(Suppl2):508A.
- Yoest J, Clark BZ, Onisko A, Dabbs DJ. Breast carcinoma biomarkers: effects of hydrochloric acid and formic acid decalcification on immunohistochemistry and in situ hybridization. 106th Annual Meeting of the USCAP; March 4–10, 2017; San Antonio. Mod Pathol. 2017;30(Suppl2):522A.
Megan Troxell, MD, PhD
Professor, Department of Pathology, Stanford University School of Medicine, Stanford, Calif.
Chair, CAP Immunohistochemistry Committee
Mohamed El-Sayed Salama, MD
Medical Director, Mayo Medical Laboratories, Mayo Clinic, Rochester, Minn.
Member, CAP Immunohistochemistry, Committee
Rajan Dewar, MD, PhD
Associate Professor, Director, Hematology Laboratory
University of Michigan, Ann Arbor
Member, CAP Surgical Pathology Committee