Editor: Frederick L. Kiechle, MD, PhD
Submit your pathology-related question for reply by appropriate medical consultants. CAP TODAY will make every effort to answer all relevant questions. However, those questions that are not of general interest may not receive a reply. For your question to be considered, you must include your name and address; this information will be omitted if your question is published in CAP TODAY.
Q. I am updating our procedure for blood draw volume limits and using So You’re Going to Collect a Blood Specimen: An Introduction to Phlebotomy, 15th edition, by Frederick L. Kiechle, MD, PhD, as a guide. The chart in the manual lists volume limits for a single blood draw at 2 cc/kg. Other charts online list 2.5 cc/kg and a maximum milliliters per 30-day period that is twice the single blood draw (5 cc/kg). I am going to use 2 cc/kg and add a column for maximum milliliters in a 30-day period at 4 cc/kg.
The phlebotomists are confused about whether a single blood draw means every day of the patient’s admission or if you would take the single blood draw and only allow the remainder of the 30-day limit. You could essentially draw the single blood draw volume limit on day one and the remainder on day two. Please clarify.
A.January 2023—This question addresses the accuracy of the table titled “Recommended volume limits for a single blood draw” on page 10 of the cited reference.1 The table uses 2 cc/kg or 2 mL/kg in a 24-hour period to determine the maximum recommended blood draw in milliliters based on the patient’s weight. Note: This maximum volume is based on a 24-hour period. The table does not define the maximum cumulative draw volume allowed per 30 days or length of hospitalization.2 Guidelines for minimal risk for pediatric blood sample volume limits range from one to five percent of total blood volume within 24 hours up to 10 percent of total blood volume over eight weeks.2 Sick children have lower limits, with a maximum of 3 mL/kg post-neonatally within 24 hours or 3.8 percent of total blood volume.2 Whole blood volume may be calculated for adults using BV = 0.3669 × h3 + 0.03219 × w + 0.6041 for men and BV = 0.3561 × h3 + 0.3308 × w + 0.1833 for women (BV = blood volume in liters, h = height in meters, w = body weight in kilograms).3 In healthy adults, the maximum blood draw should be 10.5 mL/kg or 550 mL, whichever is less over an eight-week period (https://bit.ly/UofM-drawvol).
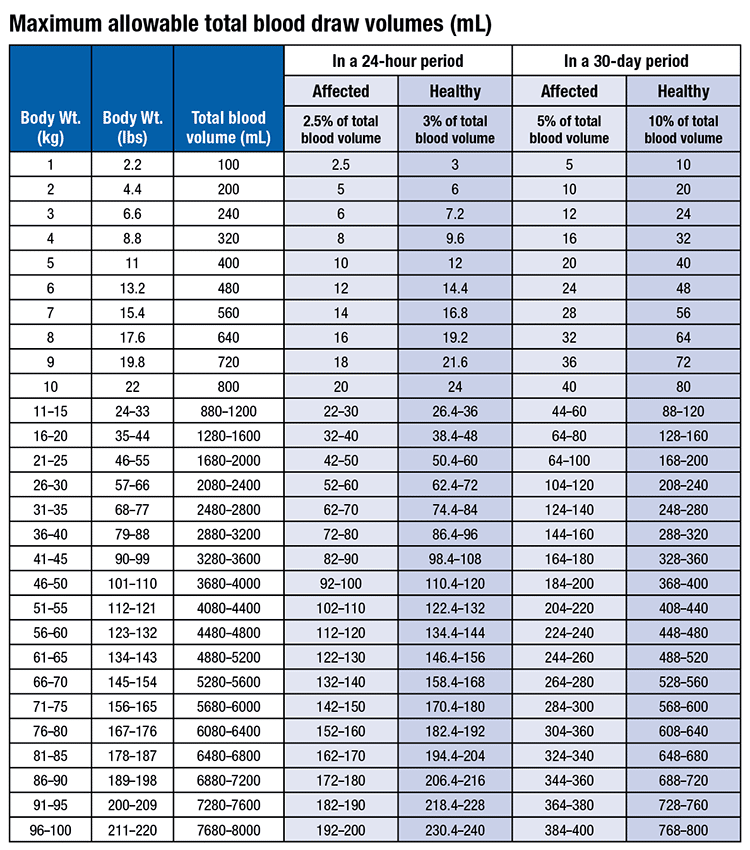 Policies and recommendations on safe blood sample volume limits for pediatric patients2 vary from 2.5 mL/kg per day (not exceeding 4 mL/kg per day) (https://bit.ly/SEAchild-drawvol), or 2.5 percent of total blood volume for sick patients or three percent of total blood volume for healthy individuals (https://bit.ly/UPenn-drawvol), or 2.4 mL/kg or three percent of total blood volume per 24-hour period.4 These recommendations will vary by practice location based on input from laboratorians, clinicians, and others.
Policies and recommendations on safe blood sample volume limits for pediatric patients2 vary from 2.5 mL/kg per day (not exceeding 4 mL/kg per day) (https://bit.ly/SEAchild-drawvol), or 2.5 percent of total blood volume for sick patients or three percent of total blood volume for healthy individuals (https://bit.ly/UPenn-drawvol), or 2.4 mL/kg or three percent of total blood volume per 24-hour period.4 These recommendations will vary by practice location based on input from laboratorians, clinicians, and others.
The table, from the University of Pennsylvania (https://bit.ly/UPenn-drawvol), is a good example of how the table in the 2017 reference1 could be modified. With no updated version of the phlebotomy manual planned, this information should be useful in developing local guidelines for maximum blood draw volumes for 24 hours or longer.
- Kiechle FL. So You’re Going to Collect a Blood Specimen: An Introduction to Phlebotomy. 15th ed. CAP Press; 2017.
- Howie SRC. Blood sample volumes in child health research: review of safe limits. Bull World Health Organ. 2011;89(1):46–53.
- Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–232.
- Peplow C, Assfalg R, Beyerlein A, Hasford J, Bonifacio E, Ziegler AG. Blood draws up to 3% of blood volume in clinical trials are safe in children. Acta Paediatr. 2019;109(5):940–944.
Frederick L. Kiechle, MD, PhD
Editor, CAP TODAY Q & A Column
Chief Medical Officer
Boca Biolistics reference laboratory
Pompano Beach, Fla.
Member, CAP Publications Committee
Q. An oncologist contacted the laboratory to ask if our standard estradiol immunoassay was appropriate to monitor her breast cancer patients who are on an aromatase inhibitor. What should I say?
A.Mass-spectrometry–based assays are preferred for measuring estradiol (E2) in populations where low concentrations are expected, such as in males, postmenopausal females, prepubertal children, and those receiving estrogen-suppressing medications or therapies. Comparing the E2 reference interval for postmenopausal females (approximately <10 pg/mL) to that of premenopausal females (15–350 pg/mL, depending on the phase of the menstrual cycle) illustrates what concentrations could be considered low.
Aromatase inhibitors (AIs) reduce the production of estrogen and are used in postmenopausal women with hormone-receptor–positive breast cancer. AI therapy can reduce already low E2 concentrations in these patients to <1 pg/mL.1,2 AI therapy failure, on the other hand, is associated with E2 concentrations in the 5–20 pg/mL range.3 Therefore, E2 is used as a potential biomarker to guide treatment decisions in these patients.
The most common methods for measuring E2 are immunoassays and liquid chromatography-mass spectrometry (LC-MS) methods. The lower limit of quantitation (LLOQ) of immunoassays is approximately 5–30 pg/mL compared to <1–5 pg/mL for LC-MS.4,5 For a laboratory to be certified by the CDC Hormone Standardization Program, the total allowable error of its estradiol assay must be ± 2.5 pg/mL for samples ≤ 20 pg/mL.6 This is problematic for immunoassays, which generally have relatively high LLOQs. In addition, immunoassays demonstrate positive bias, according to results reported in the CAP Accuracy-Based Programs Survey. Finally, immunoassays are subject to interference by drugs such as fulvestrant and the aromatase inhibitor exemestane.7,8 In summary, LC-MS assays are preferred for populations with low E2 concentrations.
- Dixon JM, Renshaw L, Young O, et al. Letrozole suppresses plasma estradiol and estrone sulphate more completely than anastrozole in postmenopausal women with breast cancer. J Clin Oncol. 2008;26(10):1671–1676.
- Handelsman DJ, Gibson E, Davis S, Golebiowski B, Walters KA, Desai R. Ultrasensitive serum estradiol measurement by liquid chromatography-mass spectrometry in postmenopausal women and mice. J Endocr Soc. 2020;4(9):bvaa086.
- Faltinová M, Vehmanen L, Lyytinen H, et al. Monitoring serum estradiol levels in breast cancer patients during extended adjuvant letrozole treatment after five years of tamoxifen: a prospective trial. Breast Cancer Res Treat. 2021;187(3):769–775.
- Bertelsen BE, Kellmann R, Viste K, et al. An ultrasensitive routine LC-MS/MS method for estradiol and estrone in the clinically relevant sub-picomolar range. J Endocr Soc. 2020;4(6):bvaa047.
- Nagao T, Kira M, Takahashi M, et al. Serum estradiol should be monitored not only during the peri-menopausal period but also the post-menopausal period at the time of aromatase inhibitor administration. World J Surg Oncol. 2009;7:88.
- HoSt/VDSCP: Certified Participants. Centers for Disease Control and Prevention. https://www.cdc.gov/labstandards/hs_certified_participants.html
- Owen LJ, Monaghan PJ, Armstrong A, et al. Oestradiol measurement during fulvestrant treatment for breast cancer. Br J Cancer. 2019;120(4):404–406.
- Mandic S, Kratzsch J, Mandic D, et al. Falsely elevated serum oestradiol due to exemestane therapy. Ann Clin Biochem. 2017;54(3):402–405.
Brian Harry, MD, PhD
Assistant Professor of Pathology
University of Colorado Anschutz Medical Campus
Aurora, Colo.
Member, CAP Accuracy-Based Programs Committee
Joely Straseski, PhD, DABCC
Professor of Pathology
University of Utah School of Medicine
Section Chief, Clinical Chemistry
Medical Director, Endocrinology
ARUP Laboratories
Salt Lake City, Utah
Member, CAP Accuracy-Based Programs Committee