Editor: Frederick L. Kiechle, MD, PhD
Submit your pathology-related question for reply by appropriate medical consultants. CAP TODAY will make every effort to answer all relevant questions. However, those questions that are not of general interest may not receive a reply. For your question to be considered, you must include your name and address; this information will be omitted if your question is published in CAP TODAY.
Q. Are there any FDA-approved laboratory-developed assays or point-of-care assays for detecting the presence of oxycodone or fentanyl in urine or blood?
A. There are two FDA-cleared instrument-based fentanyl assays for use with human urine: Fentanyl Urine Sefria Drug Screening Kit (Immunalysis) and Ark Fentanyl Assay (Ark Diagnostics).1,2 Both assays have a cutoff concentration of 1.0 ng/mL of fentanyl but have minimal reactivity with the metabolite norfentanyl. The Thermo Scientific DRI Fentanyl Assay is not FDA cleared and has a cutoff of 2.0 ng/mL of fentanyl.
As with any immunoassay-based test, users should carefully review assay package inserts for immunospecificity for possible interfering substances that give false-positive results. Equally important is how well the assays can detect fentanyl analogs, such as acetylfentanyl, arylfentanyl, and butyrfentanyl, that have been reported to be present in street drugs. Since many definitive tests for fentanyl have been developed only for fentanyl and norfentanyl, a positive fentanyl immunoassay due to the presence of a fentanyl analog may not be confirmed by the definitive test. A study comparing the three immunoassays was published in 2018.3
FDA-cleared, instrument-based urine oxycodone test kits are available from Abbott Diagnostics, Beckman Coulter, Immunalysis, Ortho Clinical Diagnostics, Roche Diagnostics, Siemens Healthineers, and Thermo Fisher Scientific. Users should review cross-reactivities of the oxycodone metabolites noroxycodone, oxymorphone, and noroxymorphone since it is possible to have low oxycodone concentrations but much higher metabolite concentrations. Good reactivity for noroxycodone and oxymorphone is useful because the mean detection times for these two oxycodone metabolites are significantly longer than that for oxycodone.4
FDA-cleared point-of-care devices for urine fentanyl or oxycodone testing are available from MedTox Diagnostics and Abbott (Alere), among others. Because these are immunoassays, the issues discussed here for instrument-based assays also apply.
Currently there are no FDA-cleared oxycodone or fentanyl assays for use with human blood.
- Fentanyl Urine Sefria Drug Screening Kit [package insert]. Pomona, Calif.: Immunalysis Corp.; 2017.
- Ark Fentanyl Assay [package insert]. Fremont, Calif.: Ark Diagnostics; 2018.
- Helander A, Stojanovik K, Villén T, Beck O. Detectability of fentanyl and designer fentanyls in urine by 3 commercial fentanyl immunoassays. Drug Test Anal. Epub ahead of print March 12, 2018. doi:10.1002/dta.2382.
- Substance Abuse and Mental Health Services Administration. Oxycodone and hydrocodone: detection in urine, oral fluid, and blood. http://bit.ly/SAMHSA-study. Published June 10, 2014.
Tai C. Kwong, PhD,
Professor, Department of Pathology and Laboratory Medicine
School of Medicine and Dentistry
Director, Clinical Chemistry Laboratory
University of Rochester (NY) Medical Center
Member, CAP Toxicology Resource Committee
A spurious spike in triglycerides
The clinical laboratory at Kaiser Permanente Northwest recently encountered a sudden shift in values in its triglyceride assay. What was the cause? Thousands of dollars and 30 hours of downtime later, the laboratory had its answer. Here it is for all.
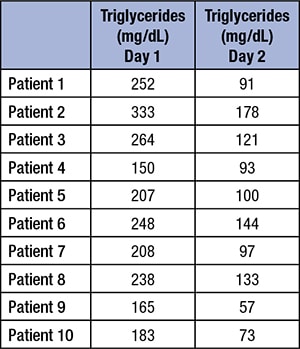 A sudden change in plasma triglycerides was noted in our laboratory over the course of one day. No other analytes were affected. Quality control results for triglycerides were within acceptable ranges at the beginning of the shift. After nearly 800 triglyceride tests were performed on the shift, QC was run again, at the start of the next workday, and was now more than four standard deviations above expected means. Extensive troubleshooting was performed, including recalibration, use of new reagent lots, and instrument service. The only abnormality identified was that the first standard (which contains only deionized water and serves as a blank) had measurable triglycerides present (twice as high as would be expected). During the troubleshooting process, QC suddenly returned to expected values. The first standard (the water blank) showed decreasing triglyceride values over the next 48 hours. Patient samples were pulled from the previous day and re-analyzed. All patient results from the previous day were high (see box). A laboratory worker serendipitously overheard that an engineer had worked on the central deionized water system in the laboratory.
A sudden change in plasma triglycerides was noted in our laboratory over the course of one day. No other analytes were affected. Quality control results for triglycerides were within acceptable ranges at the beginning of the shift. After nearly 800 triglyceride tests were performed on the shift, QC was run again, at the start of the next workday, and was now more than four standard deviations above expected means. Extensive troubleshooting was performed, including recalibration, use of new reagent lots, and instrument service. The only abnormality identified was that the first standard (which contains only deionized water and serves as a blank) had measurable triglycerides present (twice as high as would be expected). During the troubleshooting process, QC suddenly returned to expected values. The first standard (the water blank) showed decreasing triglyceride values over the next 48 hours. Patient samples were pulled from the previous day and re-analyzed. All patient results from the previous day were high (see box). A laboratory worker serendipitously overheard that an engineer had worked on the central deionized water system in the laboratory.
So, what caused the sudden shift in values seen only in the triglyceride assay?
The reverse osmosis water purification membranes were changed without informing the laboratory staff. The membranes contained glycerol as a preservative. Engineers replaced the membrane cylinders just after QC had been performed for the day. Triglyceride assays utilize the enzymatic hydrolysis of triglycerides by lipase to produce glycerol and free fatty acids.1–3 Glycerol in the lab’s water system caused an erroneous overcalculation of plasma triglycerides. The glycerol flushed from the system by day two, due to normal water usage in the laboratory. Once this was discovered, all patient samples were repeated and the results (including calculated LDL) were corrected. Since glycerol is the measurand used in most triglyceride assays, free glycerol that is present will cause a falsely elevated triglyceride concentration. This is also seen in patients with glycerol kinase deficiency, a genetic condition in which plasma glycerol levels are high and that leads to falsely elevated triglycerides when these patients have lipids measured.4
-
- McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987;166(1):1–8.
- Triglycerides/Glycerol Blanked [method sheet]. Indianapolis: Roche Diagnostics; 2018.
- Siedel J, Schmuck R, Staepels J, Town M-H. Long term stable, liquid ready-to-use mono-reagent for the enzymatic assay of serum or plasma triglycerides (GPO-PAP method). Clin Chem. 1993;39(6):1127. Abstract 34.
- Backes JM, Dayspring T, Mieras T, Moriarty PM. Pseudohypertriglyceridemia: two cases of probable glycerol kinase deficiency. J Clin Lipidol. 2012;6(5):469–473.
Kevin F. Foley, PhD, MT, DABCC
Director, Clinical Pathology
Kari Stevens, MT(ASCP)
Technologist, Core Laboratory
Krista M. Moore, MT(ASCP)
Chemistry Technical Specialist
Kaiser Permanente Northwest
Portland, Ore.