Editor: Frederick L. Kiechle, MD, PhD
Submit your pathology-related question for reply by appropriate medical consultants. CAP TODAY will make every effort to answer all relevant questions. However, those questions that are not of general interest may not receive a reply. For your question to be considered, you must include your name and address; this information will be omitted if your question is published in CAP TODAY.
Q. Should phosphate analysis be added to the comprehensive metabolic panel, especially given the test’s usefulness in distinguishing various bone disorders?
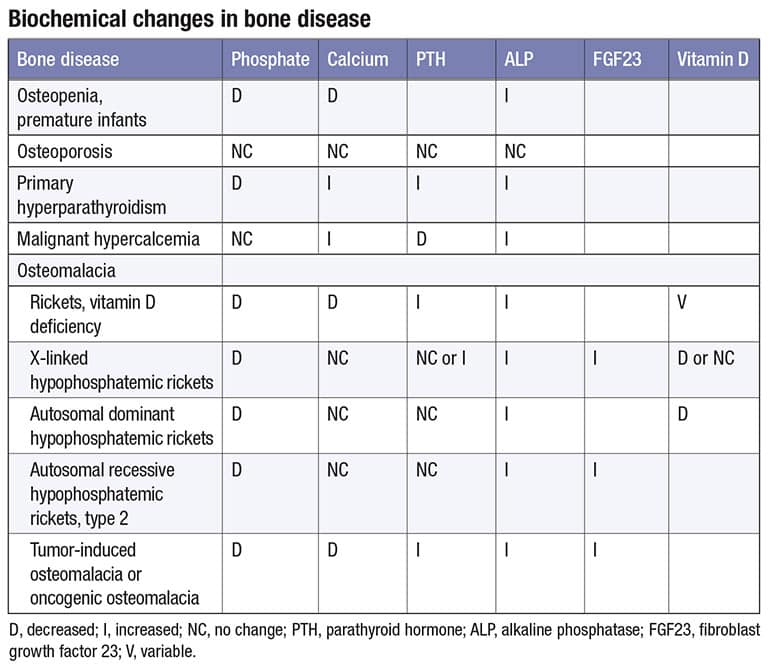
A.July 2023—Phosphate represents one percent of total body weight and has a normal reference range of 2.5 to 4.5 mg/dL. The reference range is highest in infants (4.5–8.3 mg/dL) and decreases with age. The majority of phosphate in the skeleton is in the form of hydroxyapatite crystals or amorphous calcium phosphate.1 There are two forms of phosphate in serum: dihydrogen phosphate (H2PO4) and monohydrogen phosphate (HPO4).1 The table illustrates biochemical changes in bone diseases.2,3,4
Three hormones regulate phosphate homeostasis: vitamin D (calciferol) or D2 (ergocalciferol) and D3 (cholecalciferol), which are processed by the liver to 25-hydroxy vitamin D; parathyroid hormone; and fibroblast growth factor 23. FGF23 is produced by osteocytes or osteoblasts and consists of 251 amino acids with a signal peptide comprising 24 amino acids.3,5 It inhibits renal reabsorption of phosphate after binding to the FGFR1-Klotho complex.6 Alpha-Klotho serves as a co-receptor.6 Tumor-induced osteomalacia (TIO) is a rare paraneoplastic disorder caused by benign phosphaturic mesenchymal tumors that secrete FGF23 and can present anywhere in the body. TIO is diagnosed, on average, 2.9 years after the onset of symptoms, and the underlying tumor is identified, on average, 5.4 years after symptom onset. Therefore, a chronically low phosphate found via screening may be the only clue that points to the onset of TIO.2,3,5,6
Osteopenia is a condition in which bone mineral density is lower than the normal peak density but not low enough to be considered osteoporosis.4 Several diseases or therapies have been associated with increased bone fragility and fractures, including diabetes mellitus type 17 and type 2,8 hormone treatments for transgender transition (puberty blocks and gender-affirming therapy),9 and subclinical thyroid dysfunction with a thyrotropin level of less than 0.56 mIU/L.10
There are a variety of other disorders associated with hyperphosphatemia or hypophosphatemia that would benefit from a screening phosphate determination.11,12 It is clearly time to reassess whether to include serum phosphate in the comprehensive metabolic panel.
- Qadeer HA, Bashir K. Physiology, Phosphate. In: StatPearls. StatPearls Publishing; Aug. 2022. https://www.ncbi.nlm.nih.gov/books/NBK560925
- Lee J, Vasikaran S. Current recommendations for laboratory testing and use of bone turnover markers in management of osteoporosis. Ann Lab Med. 2012;32(2):105–112.
- Li L, Wang S-X, Wu M-W, et al. Acquired hypophosphatemic osteomalacia is easily misdiagnosed or neglected by rheumatologists: a report of 9 cases. Exp Ther Med. 2018;15(6):5389–5393.
- Elam REW. Osteoporosis. Medscape. Updated Dec. 21, 2022. https://emedicine.medscape.com/article/330598-overview
- Dahir K, Zanchetta MB, Stanciu I, et al. Diagnosis and management of tumor-induced osteomalacia; perspectives from clinical experience. J Endocr Soc. 2021;5(9):bvab099.
- Kuro-o M. The Klotho proteins in health and disease. Nat Rev Nephrol. 2019;15(1):27–44.
- Napoli N, Conte C. Bone fragility in type 1 diabetes mellitus: new insights and future steps. Lancet Diabetes Endocrinol. 2022;10(7):475–476.
- Sheu A, Greenfield JR, White CP, et al. Assessment and treatment of osteoporosis and fractures in type 2 diabetes. Trends Endocrinol Metab. 2022;33(5):333–344.
- Giacomelli G, Meriggiola MC. Bone health in transgender people: a narrative review. Ther Adv Endocrinol Metab. 2022;13. doi:10.1177/20420188221099346
- Daya NR, Fretz A, Martin SS, et al. Association between subclinical thyroid dysfunction and fracture risk. JAMA Netw Open. 2022;5(11):e2240823.
- Goyal R, Jialal I. Hyperphosphatemia. In: StatPearls. Stat Pearls Publishing, June 2023. https://www.ncbi.nlm.nih.gov/books/NBK551586
- Lewis JL. Hypophosphatemia. Merck Manual. Published Sept. 2021. Updated Sept. 2022. https://www.merckmanuals.com/en-ca/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/hypophosphatemia
Frederick L. Kiechle, MD, PhD
Editor, CAP TODAY Q & A Column
Chief Medical Officer
Boca Biolistics reference laboratory
Pompano Beach, Fla.
Member, CAP Publications Committee
Q. Is it important to fast before a lipid panel?
A.Cholesterol and other lipids are transported in plasma by various lipoprotein particles, including low-density lipoproteins (LDL) and high-density lipoproteins (HDL). While HDL is non-atherogenic and may even be protective, an elevated plasma concentration of the cholesterol carried in LDL (LDL-C) is a well-described risk factor for atherosclerotic cardiovascular disease (ASCVD).
Clinical practice guidelines recommend a target LDL-C level of <100 mg/dL for the primary prevention of ASCVD.1 Importantly, LDL-C is not typically measured directly in clinical practice. It is more often calculated using the Friedewald formula (calculated LDL-C = total cholesterol − HDL-C − triglycerides/5). Fasting values historically have been recommended to ensure that triglycerides are <400 mg/dL and that accurate calculated LDL-C values are obtained. However, recent studies have confirmed that nonfasting calculated LDL-C values have worth in predicting ASCVD.2,3 Yet it is now widely recommended that nonfasting non–HDL-C be used for patient management in the general population.
Non–HDL cholesterol, which is calculated by subtracting HDL-C from total cholesterol, encompasses all atherogenic lipoprotein particles and more effectively predicts the risk of major cardiovascular events, including coronary artery disease and stroke, than does LDL-C.4,5 Additionally, in contrast to LDL-C and triglyceride levels, total cholesterol and HDL-C levels are minimally affected by fasting status.2,3 Therefore, non–HDL-C can be reliably calculated from nonfasting specimens and the specimens of people with hypertriglyceridemia.
Although cholesterol-reducing agents originally targeted calculated LDL-C, they are now also recommended for managing non–HDL-C in the primary and secondary prevention of ASCVD.6 The target non–HDL-C levels for primary and secondary prevention are <130 mg/dL and <100 mg/dL, respectively. These cutoffs are suitable for fasting and nonfasting specimens.7
Implementation of non–HDL-C testing in the clinical laboratory involves a simple calculation and no additional assays, and it supports patient care by being convenient for all and ideal for those in whom fasting carries additional risk. Measurement of non–HDL-C is thus simple, robust, and clinically effective, and clinical laboratories should make it widely available for assessing ASCVD risk.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139(25):e1082–e1143.
- Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118(20):2047–2056.
- Sidhu D, Naugler C. Fasting time and lipid levels in a community-based population: a cross-sectional study. Arch Intern Med. 2012;172(22):1707–1710.
- Boekholdt SM, Arsenault BJ, Mora S, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA. 2012;307(12):1302–1309.
- Brunner FJ, Waldeyer C, Ojeda F, et al. Application of non-HDL cholesterol for population-based cardiovascular risk stratification: results from the Multinational Cardiovascular Risk Consortium. Lancet. 2019;394(10215):2173–2183.
- Expert Dyslipidemia Panel, Grundy SM. An International Atherosclerosis Society position paper: global recommendations for the management of dyslipidemia. J Clin Lipidol. 2013;7(6):561–565.
- Guo LL, Chen YQ, Lin QZ, et al. Non-HDL-C is more stable than LDL-C in assessing the percent attainment of non-fasting lipid for coronary heart disease patients. Front Cardiovasc Med. 2021;8:649181.
Andy Hoofnagle, MD, PhD
Professor, Laboratory Medicine
Head, Division of Clinical Chemistry
Department of Laboratory Medicine and Pathology
University of Washington, Seattle, Wash.
Chair, CAP Accuracy-Based
Programs Committee
Rebecca Treger, MD, PhD
Clinical Pathology Resident
University of Washington, Seattle, Wash.
Junior Member, CAP Accuracy-Based Programs Committee
Submit your pathology-related question
Submit your pathology-related question for reply by appropriate medical consultants. CAP TODAY will make every effort to answer all relevant questions. However, those questions that are not of general interest may not receive a reply. For your question to be considered, you must include your name and address; this information will be omitted if your question is published in CAP TODAY.