Editor: Frederick L. Kiechle, MD, PhD
Submit your pathology-related question for reply by appropriate medical consultants. CAP TODAY will make every effort to answer all relevant questions. However, those questions that are not of general interest may not receive a reply. For your question to be considered, you must include your name and address; this information will be omitted if your question is published in CAP TODAY.
Q. What is the total allowable error for lupus anticoagulant testing?
A.October 2023—Total allowable error is the maximum amount of error, combining bias and imprecision, that is deemed acceptable for an assay. It can be defined by regulatory agencies or calculated as 0.25 × (CVi2 + CVg2)0.5 + z × 0.5 × CVi, where CVi means within-subject coefficient of variation, CVg means between-subject coefficient of variation, and z represents the z-score of a desired confidence limit (for example, z = 1.65 for a one-sided 95 percent confidence interval).
Published total allowable error (%) for common coagulation analytes are as follows:
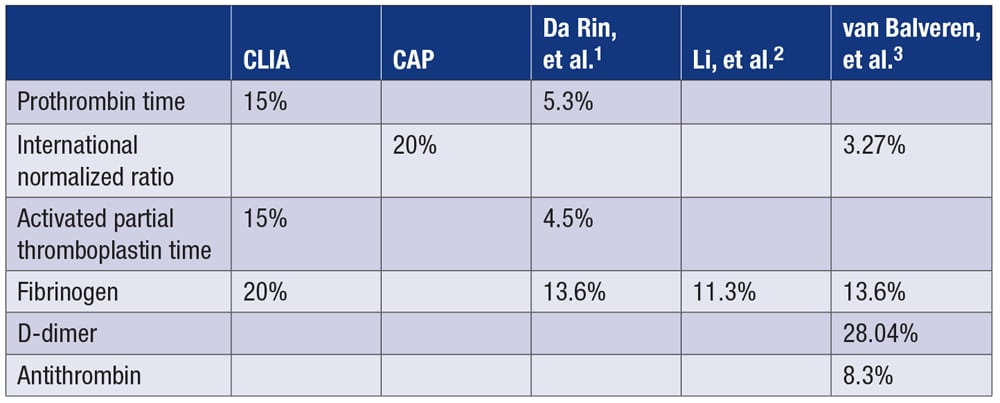
The European Federation of Clinical Chemistry and Laboratory Medicine and Westgard QC do not include lupus anticoagulants in their analyte variation databases.4,5 Despite updating its guidelines for lupus anticoagulant detection and interpretation, the International Society on Thrombosis and Haemostasis scientific and standardization committee’s subcommittee on lupus anticoagulant/antiphospholipid antibodies does not provide total allowable error recommendations for lupus anticoagulant.6
To our knowledge, only one article, by Shou, et al., has reported the within-subject and between-subject coefficients of variation of lupus anticoagulant, but it was specifically for dilute Russell viper venom time (DRVVT) using an Instrumentation Laboratory device.7 Based on those authors’ data, total allowable error can be calculated as follows:

Once the total allowable error for an analyte is defined, it can be used as a benchmark for gauging a test’s performance quantified by its total analytical error. The total analytical error for an assay can be calculated by combining the estimate of bias from a method-comparison study and the estimate of imprecision from a replication study: total analytical error = bias + 2 × standard deviations.8,9
- Da Rin G, Lippi G. Total laboratory automation of routine hemostasis testing. J Lab Autom. 2014;19(4):419–422.
- Li C, Sun Z, Liu Y, Zhou W, Wang Y, Peng M. Comparison among different measurement systems for fibrinogen using fresh samples and frozen samples. Clin Chim Acta. 2020;509:258–263.
- van Balveren JA, Huijskens MJ, Gemen EF, Péquériaux NC, Kusters R. Effects of time and temperature on 48 routine chemistry, haematology and coagulation analytes in whole blood samples. Ann Clin Biochem. 2017;54(4):448–462.
- Aarsand AK, Fernandez-Calle P, Webster C, et al. The EFLM biological variation database. European Federation of Clinical Chemistry and Laboratory Medicine. https://biologicalvariation.eu/
- Desirable biological variation database specifications. Westgard QC. www.westgard.com/biodatabase1.htm
- Devreese KMJ, de Groot PG, de Laat B, et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis: update of the guidelines for lupus anticoagulant detection and interpretation. J Thromb Haemost. 2020;18(11):2828–2839.
- Shou W, Chen Q, Wu W, Cui W. Biological variations of lupus anticoagulant, antithrombin, protein C, protein S, and von Willebrand factor assays. Semin Thromb Hemost. 2016;42(1):87–92.
- Westgard JO, Westgard SA. The quality of laboratory testing today: an assessment of sigma metrics for analytic quality using performance data from proficiency testing surveys and the CLIA criteria for acceptable performance. Am J Clin Pathol. 2006;125(3):343–354.
- Westgard JO, Westgard SA. Total analytical error: from concept to application. Clinical Laboratory News. Sept. 1, 2013. www.aacc.org/cln/articles/2013/september/total-analytic-error
Geoffrey Wool, MD, PhD
Associate Professor
Department of Pathology
Medical Director, Section of Transfusion, Hemostasis, and Apheresis Medicine
Director, Transfusion Medicine
Fellowship Program
University of Chicago
Chicago, Ill.
Member, CAP Hemostasis and Thrombosis Committee
Clarence Chan, MD, PhD
Chief Resident, Clinical Pathology
Department of Pathology
University of Chicago
Chicago, Ill.
Junior Member, CAP Hemostasis and Thrombosis Committee
Q. Our laboratory may relocate to a building five blocks from our current hospital. What kind of instrument validation or verification studies do we need to perform following a move? When should we update the address on our CLIA license and for CAP accreditation? Are we required to have a new CAP inspection before or after testing patient samples at the new location?
A.Numerous factors must be considered when moving a laboratory. Will the laboratory retain its current CLIA certificate? Are there organizational changes that necessitate getting a new CLIA certificate (for example, a change in ownership)? How long will instruments be out of use? Will the laboratory retain the same personnel? Will there be significant changes to the laboratory environment?
If the Centers for Medicare and Medicaid Services requires the laboratory to obtain a CLIA certificate for its new location, the laboratory is considered new and will need to perform initial test-method validation or test-method verification for all tests.
A laboratory that is able to retain its CLIA certificate when it moves is responsible for ensuring that method-performance specifications, such as accuracy and precision, are not affected by the relocation process or the new environment. The laboratory should follow the instrument manufacturer’s instructions for setup, maintenance, and system verification. Laboratories must verify operational performance to ensure instruments are performing according to manufacturer and laboratory specifications. The CAP recommends that the laboratory consult with the instrument or equipment manufacturer for guidance.
Some manufacturers require that a service representative verify that instruments are functioning appropriately following a move. If the instrument manufacturer does not provide guidance on how to verify test performance following a move, the laboratory must determine the extent of the validation and verification studies needed.
Another factor to consider when determining the extent of validation or verification needed is the risk of damage to the instrument during the relocation process. Moving an instrument a few inches or a few feet within the laboratory may have minimal risk and require less extensive validation or verification compared with moving an instrument several blocks. Instruments that are out of service for long periods of time due to a move may need to be reconditioned, and laboratory personnel may need to be retrained on the instrument or undergo competency assessment.
Laboratories should perform function checks and quality control prior to testing patient samples on an instrument that has been moved, and they should verify the calibration of each assay and recalibrate as necessary. Laboratories should also conduct comparison and repeatability studies using patient samples tested before a move to ensure the assays continue to be accurate and precise. The laboratory director, or a designee meeting CAP director qualifications, must review the data from those studies and approve each assay for use prior to patient testing. The laboratory must retain these records for the time the test is in use plus two years.
A laboratory must update its address with its state CLIA office within 30 days of a move. It must also promptly notify the CAP of the new address by updating the address at www.cap.org. (Go to Access e-LAB Solutions Suite at the top of the homepage. Then click the Organization Profile submenu.) The CAP will review the address update and any other changes to determine if an inspection or other action is required to continue the laboratory’s accreditation.
Clinical and Laboratory Standards Institute. EP05-A3: Evaluation of Precision of Quantitative Measurement Procedures; Approved Guideline. 3rd ed. CLSI; 2014.
Clinical and Laboratory Standards Institute. EP09c: Measurement Procedure Comparison and Bias Estimation Using Patient Samples. 3rd ed. CLSI; 2018.
Clinical and Laboratory Standards Institute. C24: Statistical Quality Control for Quantitative Measurement Procedures: Principles and Definitions. 4th ed. CLSI; 2016.
College of American Pathologists. All common checklist. Oct. 24, 2022.
Sara Lieske, MPH, CLS(ASCP)
Checklist Technical Content Analyst
College of American Pathologists
Northfield, Ill.