Amy Carpenter Aquino
September 2019—The results of a clinical trial of a rapid assay for dual detection of HIV and syphilis were reported at the 2019 HIV Diagnostics Conference in March. If approved by the FDA, where it has been under consideration since 2018, the Chembio Diagnostic Systems assay would be the first point-of-care assay of its kind on the U.S. market.
“If we’re going to address the situations of infectious diseases throughout the world for most of the people in this world, we’re going to have to use rapid assays,” said Niel T. Constantine, PhD, MT(ASCP), professor of pathology, Institute of Human Virology, University of Maryland School of Medicine.

Dr. Constantine
Dr. Constantine, who is director of the institute’s laboratory of viral diagnostics, presented the findings of his evaluation of the Dual Path Platform HIV-Syphilis assay and Micro Reader, which provide results in 10 to 30 minutes with fingerstick, venous whole blood, or plasma.
For syphilis detection, a rapid assay with robust storage of up to 30°C is particularly advantageous, Dr. Constantine said, since the reference tests for syphilis—RPR (rapid plasma reagin) and TP-PA (T. pallidum particle agglutination)—require cold chain shipment and refrigeration. He called rapid tests for syphilis “probably the only appropriate tests that can be used in many places throughout the world.”
In 2015, the Standard Diagnostics Bioline HIV/Syphilis Duo assay, which is not available in the U.S., became the first HIV-syphilis multiplex test placed on the WHO list of prequalified in vitro diagnostic products. “Their evaluation showed pretty low sensitivity for syphilis at 87 percent,” Dr. Constantine said. “However, they felt it was important to have this in the market, mostly for pregnant women.” WHO recommends HIV and syphilis testing for a woman at least once, preferably in the first trimester, and backs the use of a dual HIV/syphilis rapid diagnostic test to “facilitate implementation of this recommendation.”
The 2017–2018 clinical trial of the Chembio DPP HIV-Syphilis system was part of the premarket approval Chembio submitted, Dr. Constantine said. He reported the results of one portion of the trial, which consisted of testing samples from two sites: the University of Maryland and the Fanno Creek Clinic in Portland, Ore. Data from the two sites were combined to show performance characteristics.
The DPP HIV-Syphilis kit box has 20 sample collection loops to collect about 10 µL of sample from fingerstick, plasma, or venous whole blood. A buffer vial dilutes the sample, and then the sample in the sample vial is added to the device. “It’s a typical lateral flow-type assay,” Dr. Constantine said. “In this case, it’s two wells with a dual path.” The sample-buffer combination goes in the bottom well, and after five minutes, four drops of buffer are placed in the upper well. “And you read the results.”
Results must be read with the DPP Micro Reader, which sits on top of the lateral flow device and reads the result in three seconds. “Press a button and get a result for HIV. Press it again and get a result for syphilis. Simple to use, very easy, very objective,” he said. “When faint lines occur, the DPP Micro Reader takes the guessing out of the equation.”
During the study, four tubes of blood were collected from each subject. “We had to centrifuge at least one of the tubes within two hours,” Dr. Constantine said, “and that was shipped to a reference lab for reference testing of HIV and syphilis.” Fingerstick, whole blood, and plasma were tested on site with the DPP HIV-Syphilis assay.
For HIV reference testing, the central laboratory used the CDC recommended algorithm, beginning with the fourth-generation Abbott Architect HIV Ag/Ab Combo assay, followed by either the Bio-Rad Multispot HIV-1/HIV-2 Rapid differentiation test or the Bio-Rad Geenius HIV 1/2 Supplemental Assay. If those results were discordant, RNA testing was done with the Hologic Aptima HIV-1 RNA Qualitative Assay.
For syphilis screening, the central lab used the Bio-Rad BioPlex 2200 Syphilis IgG kit for detection of treponemal antibodies, and a syphilis RPR assay for detection of nontreponemal antibodies. If results were discordant, a syphilis TP-PA test was done, the results of which were definitive, Dr. Constantine said.
Eight hundred three samples were tested—324 from the University of Maryland and 479 from Fanno Creek Clinic. The 803 samples came from people known to be positive for HIV, known to be positive for syphilis, and known to be co-infected with HIV and syphilis, and from high-risk people of unknown status and low-risk individuals.
Reference test results found 351 samples to be positive for HIV, 119 to be positive for syphilis, and 91 to be co-infected. Four hundred twenty-seven were negative for HIV; 672 were negative for syphilis.
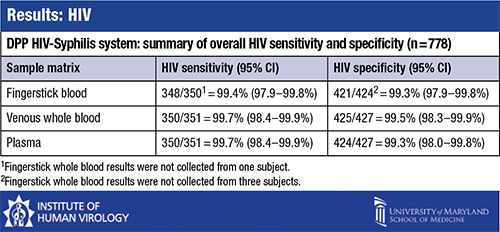
The comparison of the DPP HIV-Syphilis test results with the reference test results found that “for HIV, the test indices are about what you would expect and are excellent and sufficient, as most tests are for HIV,” Dr. Constantine said. For 778 samples, the HIV sensitivity was 99.4 percent for fingerstick blood and 99.7 percent for venous whole blood and plasma. Specificity was between 99.3 and 99.5 percent for fingerstick, whole blood, and plasma.
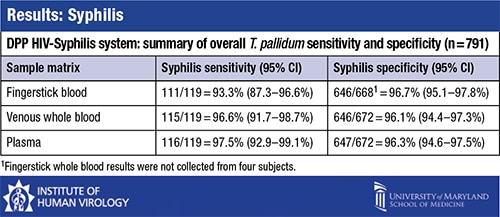
“For syphilis, sensitivity was a little less heavy depending on the matrix used, with fingerstick giving the lowest sensitivity,” at 93.3 percent, Dr. Constantine said. For venous whole blood the sensitivity was 96.6 percent; for plasma the sensitivity was 97.5 percent. Specificity was between 96.1 and 96.7 percent for all matrices. “If you average this out, it comes out to about 95 percent sensitivity for syphilis. Specificity is 96 percent or higher.”
Dr. Constantine suggested that seroreversion could have led to the false-negative TP-PA test results, “especially if a person is treated very early on during primary syphilis. Antibodies tend to not evolve as well, so that is responsible in some cases for false-negatives. The WHO also mentions that reason.” The false-negative results were found in patients who were previously diagnosed with syphilis. The degree of false-negatives between the DPP HIV-Syphilis system and the BioPlex 2200 Syphilis IgG kit was “essentially identical,” he said, at 79.4 percent.
In the syphilis reactive samples, there was good correlation between results from the DPP Micro Reader and the RPR titers.
What is the future of the DPP Micro Reader? “They tell me this is applicable,” he said, “for electronic transmission to smartphones, tablets, and other devices.”
Chembio is awaiting the FDA’s decision.
Amy Carpenter Aquino is CAP TODAY senior editor.