CAP Press released late last year its Semen Analysis Benchtop Reference Guide, an illustrated guide with emphasis on sperm morphology. Its 68 laminated pages are divided into sections: specimen collection and macroscopic assessment, sperm count, sperm morphology, and nonsperm cells.

Dr. Nelsen
Behind the book is the CAP Reproductive Medicine Committee, in particular Erica J. Behnke, PhD, HCLD, of Ohio Fertility Providers, Jacob F. Meyer Jr., PhD, HCLD, of Eastern Virginia Medical School, and Laura L. Nelsen, MD, pathologist and director of cytology at MaineGeneral Medical Center and past chair of and now advisor to the committee. CAP TODAY asked Dr. Nelsen about the benchtop reference guide, sample pages of which appear below. Here is what she told us.
Who are the intended readers of the reference guide and how will it be of use to them?
March 2019—This reference guide is aimed at laboratory technicians and scientists, either working in a reproductive laboratory or general clinical laboratory and seeking assistance on performing semen analysis. Currently, the CAP accredits 343 reproductive laboratories (of 465 registered by CMS), with many of those labs offering semen analysis. Many general labs and some urologists also offer this test to patients, which can be fraught with subjective variation, especially in the qualitative components, such as semen morphology or viability. This test is classified as high complexity by CLIA ’88, making it subject to various QA and QC requirements.
Interestingly, many people do not know about the strong collaboration between the CAP and reproductive laboratories. When I have attended the CAP Council on Scientific Affairs meetings, I usually meet at least one pathologist serving as a committee chair who is surprised to learn of our committee’s existence, as most reproductive medicine is assumed to be under the purview of obstetrical medicine and the Society for Assisted Reproductive Technology. The field of reproductive medicine is relatively young, however, and the CAP has long set the standard for lab accreditation and good practices. When commercial reproductive labs took off in the 1990s, a collaboration between our societies was formed.
How does this book differ from others on the market?
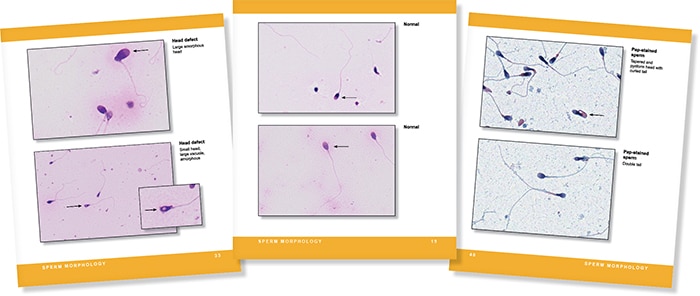
When our group was exploring the need for this reference guide, we searched for comparable benchside publications and had difficulty finding any currently available. For the majority of labs, the gold standard in analysis is the WHO laboratory manual for the examination and processing of human semen. The most current edition, the fifth, was published in 2010. Our book generally follows the WHO recommendations but as an abbreviated, illustrated guide. The WHO publication offers a comprehensive and detailed guide for performing and interpreting semen analysis, and while we recommend it to all our labs, it can be unwieldy to use on a day-to-day basis. Additionally, with more than 20 years of proficiency testing challenges, our committee had a rich library of images to draw from, to illustrate normal and abnormal spermatozoa. The large images on laminated pages that can be beside the microscope are an important tool in practice and training.
You and your coauthors write in the guide that a great debt is owed to Thinus Kruger, MB ChB, MD, of Tygerberg, South Africa, father of the modern morphologic evaluation known as Strict criteria. Can you tell us what his contribution to the benchtop guide was?
As a resident I was able to participate in an international research study and was introduced by email to Dr. Kruger, whose work has set the foundation for modern semen analysis. Dr. Kruger generously reviewed the images we provided of normal spermatozoa. Because the criteria he established—also known as Strict or Tygerberg criteria—are very specific regarding size, shape, and morphology of spermatozoa, most sperm on a stained slide are correctly classified as abnormal. In general, a typical semen analysis should not have greater than 25 percent normal sperm and, per WHO’s fifth edition, is only considered abnormal if less than four percent normal forms are present, approximately one in 20 sperm—much lower than the previous limit of 12 percent normal forms present. Different labs have set or chosen to follow different criteria, however, and our review of laboratory performance in QC for semen analysis shows a wide coefficient of variation in our Surveys of sperm morphology, often greater than 70 percent. This guide includes a short chapter on the different methodologies for reporting morphology, which explains some of this variation.
You also write that there is still wide variation between laboratories and observers despite the field of semen evaluation having evolved during the past three decades. Can you explain this variation and why accuracy is so important?
The variation between labs can be unsettling when initially reviewing the lab Survey results; how can one lab call 90 percent of the sperm normal when another lab reviewing the same specimen classifies only nine percent as normal? The reference guide devotes a chapter to the different classification systems used in reporting morphology and why that variation is present. We encourage labs to use the most recent guidelines from the WHO but do not compel them to do so. Regardless of the system used, it is important for labs to report which classification system they are using and the reference values the laboratory established.
How is the guide organized and why, and what might the reader want to know about the images?
The guide is organized by the steps in the procedure with short sections for each reportable parameter in a typical semen analysis: liquefaction, volume, viscosity, pH, color, count, motility, viability, and morphology. Many stained images of normal and abnormal spermatozoa are included for reference. Feedback from reproductive laboratories we have surveyed included a strong interest to help in classifying non-semen cells present in semen, so many pictures of spermatids (precursors to spermatozoa), epithelial cells, and inflammatory cells are also included.
To order ($89), call the CAP at 800-323-4040 option 1 (item SABRG). If you are interested in writing a book for publication by CAP Press, contact Caryn Tursky at ctursky@cap.org.